12.2 Vitamins as Electron Carriers
Water-soluble vitamins (e.g., vitamin B complex) are the precursor of several coenzymes.
The three classes of vitamin-derived coenzymes that are used to carry electrons are:
- Ascorbic acid (i.e., Vitamin C)
- Nicotiamide coenzymes (i.e., Vitamin B3 [i.e., niacin])
- Flavin coenzymes (i.e., Vitamin B2 [i.e., riboflavin])
12.2.1 Vitamin C
In its active form, vitamin C is referred to as ascorbate.
Vitamin C is an antioxidant: it inactivates free oxygen radicals and protects other antioxidants (e.g., vitamins A and E). It is a coenzyme in hydroxylation reactions (e.g., collagen synthesis) and also required for the enzyme dopamine \(\beta\)-hydroxylase (essential for norepinephrine and epinephrine synthesis). The vitamin is also essential for bile acid synthesis and tyrosine degradation.
Vitamin C is essential for iron absorption and iron reduction from Fe3+ to Fe2+.
12.2.1.1 Forms of vitamin C
Figure 12.6: States of Vitamin C
Vitamin C exists in two different forms: L-ascorbic acid (i.e., the reduced form) and L-dihydroascorbic acid (i.e., the oxidized form) - this can be seen in figure 12.6.
When ascorbic acid is hydrated, it is inactive. Nonetheless, both the reduced and oxidized forms of ascorbic acid are biologically active. The reduced form of ascorbic acid predominates in plasma and tissues at a ratio of 15 : 1 of the oxidized form.
12.2.1.2 Vitamin C recycling mechanism
Figure 12.7: Vitamin C Recycling Mechanism
In humans and other mammals that have lost the ability to synthesize ascorbate, they have a compensatory vitamin C recycling mechanism.
In figure 12.7, after ascorbic acid is used to reduce some oxidized substance, ascorbic acid is then oxidized to dehydroascorbic acid (which can be reduced to ascorbic acid).
The mechanism in figure 12.7 is key to red blood cells!
12.2.2 Vitamin B3 (niacin)
Figure 12.8: NAD and NADP with Vitamins
Niacin is a name for nicotiamide and nicotinic acid. Both molecules can also act as a precursor of the nicotiamide coenzymes NAD+ (i.e., nicotiamide adenine dinucleotide) and NADP+ (i.e., nicotiamide adenine dinucleotide-2’-phosphate).
The vitamin B3 moieties of NAD+ and NADP+ are circled in blue in figure 12.8.
12.2.2.1 Vitamin B3 oxidation and reduction
The NAD+ / NADH redox couple (see figure 12.9) is known as a regulator of cellular energy metabolism (i.e., of glycolysis and oxidative phosphorylation in the mitochondria).
Figure 12.9: Redox States of NADP
NADP+ and NADPH, on the contrary, is involved in maintaining redox balance and supporting the biosynthesis of fatty acids and nucleic acids.
While NADH is generally involved in catabolic reactions (i.e., reactions that break down molecules to release energy), NADPH is involved in anabolic reactions (i.e., reactions that consume energy to build larger molecules). When either NADP+ or NAD+ is reduced, its nicotiamide ring is involved.
Figure 12.10: Reduced and Oxidized States of NADP / NAD
The reduction of NAD+ or NADP+ (see figure 12.10) converts the benzenoid ring of the nicotiamide to the quinonoide form.
12.2.2.2 Absorbance of vitamin B3
Figure 12.11: Absorbance of NAD and NADH
NADH absorbs light at about 340 nm - NAD+ does not absorb light at all. Hence, this difference in absorption (shown in figure 12.11) is used by biochemists to assay reactions involving these coenzymes.
More than 200 enzymes are known to catalyze reactions whereby NAD+ (or NADP+) accepts a hydride ion from a reduced substrate or NADPH (or NADH) donates a hydride ion to an oxidized substrate.
A lack of niacin in one’s diet affects all NAD+ and NADP+-dependent dehydrogenases and causes a disease called pellagra.
12.2.3 Vitamin B2
Vitamin B2 (or Riboflavin) is synthesized by bacteria, fungi, plants, and some animals. All mammals obtain vitamin B2 through their diets.
Figure 12.12: Structure of Vitamin B2
Riboflavin (seen in figure 12.12) is made from the ten-carbon alcohol ribitol; the ribitol is linked to the N-10 carbon of isoalloxazine.
Vitamin B2 is involved in a variety of metabolic processes including:
- Redox reactions in the Krebs’ cycle
- Respiratory chain in the mitochondria
- Fatty acid oxidations
- Amino acid metabolism
Figure 12.13: Structure of FMN and FAD
This vitamin is also a precursor of the flavin coenzymes FMN (i.e., flavin mononucleotide) and FAD (i.e., flavin adenine dinucleotide). A flavoenzyme is an enzyme that uses flavins to carry out their reactions.
12.2.3.1 Oxidation and reduction of vitamin B2
Like vitamin B3, vitamin B2 also undergoes two-electron oxidation and reduction pathways. However, vitamin B2 has a semiquonine free radical (seen in figure 12.14) - a stable one-electron reduced species:
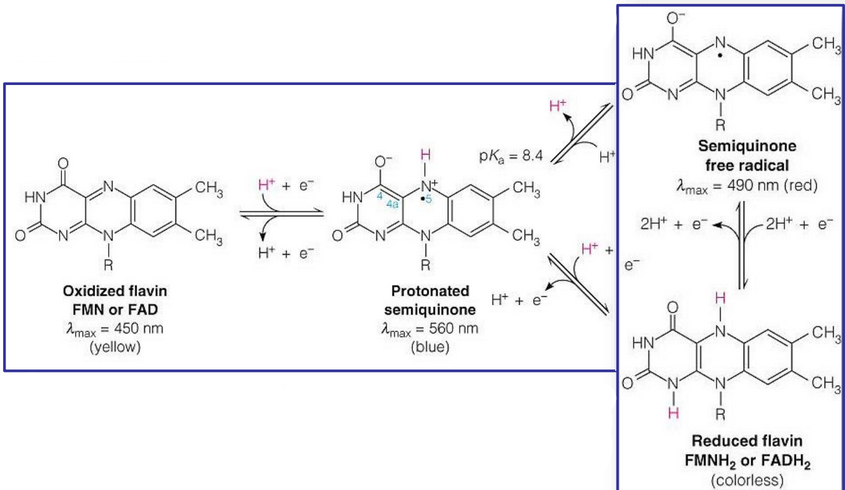
Figure 12.14: A Semiquinone Free Radical
This free radical can be detected spectrophotometrically. Whereas FAD and FMN are bright yellow (and their fully reduced counterparts are colorless), the semiquinone intermediate is either red or blue (depending on the pH of the solution).