10.3 Fatty Acid Rancidity
Rancidity refers to the spoiling of fats via oxidation. When a fatty acid has more double bonds, it is more susceptible to oxidation. Hence, polyunsaturated fatty acids are more prone to oxidation than monounsaturated fatty acids or saturated fatty acids.
The stability of fatty acids can be enhanced via adding antioxidants, limiting food exposure to light, heat, and light, and hydrogenation.
Figure 10.14: Hydrogenation of an Unsaturated Fatty Acid
Hydrogenation is a process of adding hydrogen to the carbon atoms of double bonds, hence creating saturated fatty acids in the process (see figure 10.14). Hydrogenation produces solids like margarine and shortenings.
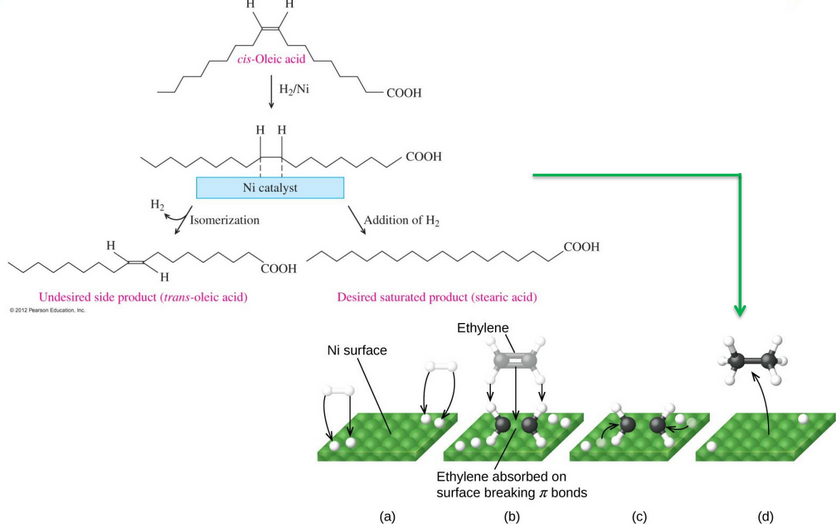
Figure 10.15: Detailed Schematic of Hydrogenation
A more detailed schematic of hydrogenation is shown in figure 10.15.
10.3.1 Types of rancidity
There are three main kinds of fatty acid rancidity:
Oxidative rancidity
This is also known as the autooxidation of a polyunsaturated fatty acid by atmospheric O2 on free radicals. Here, the polyunsaturated fatty acid reacts with oxygen to form peroxides and epoxides, forming aldehydes, ketones, and other volatile products.
Hydrolytic rancidity
This is also known as hydrolysis / enzymatic oxidation. It is due to the contamination of fat by lipase, hence leading to the formation of diacyls and monoacylglycerols with free fatty acids.
This kind of rancidity results in the formation of epoxides and peroxides.
Microbial rancidity
This is a kind of rancidity where microbes use their enzymes to catabolize the chemical structure of oils, hence producing unwated flavors and odors.
This generally occurs in the presence of water and can be prevented via sterilization.
10.3.2 Hydrolysis
Figure 10.16: Hydrolysis of a Triacylglycerol
Figure 10.16 presents the hydrolysis of a triglyceride by lipases. Three molecules of fatty acids and one molecule of glycerol is produced from hydrolysis.
Figure 10.17: Saponification Reaction
The hydrolysis of a triglyceride can also be mediated by a base (e.g., NaOH). Saponification is the simultaneous hydrolysis and neutralization of the triglyceride and the base to yield soap (see figure 10.17).
10.3.3 In summary…
Figure 10.18: Summary of Fatty Acid Reactions
Figure 10.18 summarizes the information presented in the previous subsections.