1.2 Biochemistry as a Biological Science
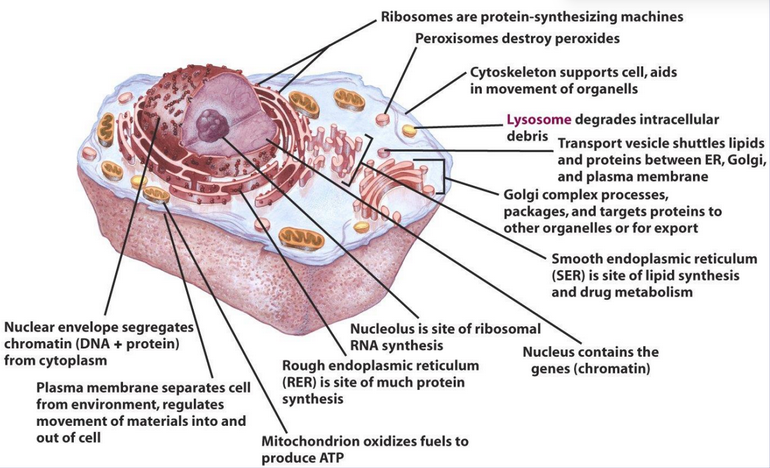
Figure 1.6: Anatomy of an Animal Cell
As shown in figure 1.6, there are many organelles (and hence functions) in an animal cell.
Figure 1.7: Levels of Biochemistry
Nonetheless, biochemistry also comes in all shapes and sizes as displayed in figure 1.7!
1.2.1 Types of polymers
There are many types of natural polymers present in the Biology side of Biochemistry.
These polymers include:
Cellulose
Figure 1.8: Structure of Cellulose
This is made by joining many identical glucose units via \(\alpha\)(1, 4) glycosidic bonds.
Starch
Like cellulose, starch is a homopolymer of glucose (i.e., a polymer made up of identical subunits). However, \(\alpha\)(1, 6) and \(\alpha\)(1, 4) glycosidic bonds are present in starch (i.e., the polymer is branched).
Polysaccharides can also serve as structural components and as a form of energy storage (e.g., glycogen).
Proteins
Figure 1.9: Structure of a Protein
These are heteropolymers of amino acids - they are linked by peptide bonds. Proteins can serve as enzymes, hormones, receptors, and structural components (e.g., collagen).
As an example, myoglobin (an oxygen-carrying protein in muscle) has a mass of 16.7 kDa. It is also distantly related to hemoglobin in red blood cells, contains iron, and binds to oxygen.
Nucleic acids
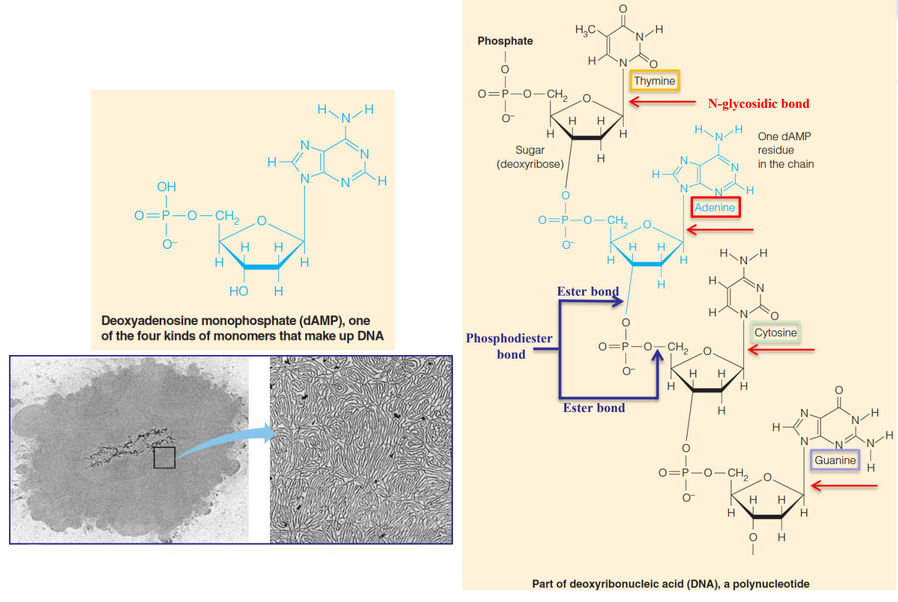
Figure 1.10: Structure of DNA
This is also a heteropolymer; it is made by combining different nucleotides: adenine, guanine, cytosine, thymine, or uracil. These polymers are responsible for the storage and the transmission of genetic information (i.e., the central dogma).
1.2.2 Lipids
Figure 1.11: Structure of Lipids
As witnessed in figure 1.11, lipids can take many forms.
Figure 1.12: Structure of a Phospholipid Bilayer
Lipids are generally used for energy storage purposes (e.g., body fat) and are the main constituents of the cell membrane.
1.2.3 Water in Biology
Figure 1.13: Structure and Intermolecular Bonding Water Molecules
As implied in figure 1.13, the oxygens in a water molecule have a greater partial negative charge (i.e., \(\delta-\)); the hydrogens have a greater partial positive charge (i.e., \(\delta+\)).
Water serves intracellular and extracellular roles due to its two properties:
- Its ability to form hydrogen bonds.
- Its polar character.
Substances that readily dissolve in water are termed hydrophilic or “water-loving.”
Figure 1.14: Hydration Shell Around an Anion
When ionic compounds are dissolved in water, the interactions of the negative ends of the water dipoles with cations and the positive ends with anions cause the ions to become hydrated. They become surrounded by water molecules and form a hydration shell (as depicted in figure 1.14).