6.1 Components of Nucleotides
All nucleotides have three components (as seen in figure 6.2); they have…
a nitrogen-containing base
In a DNA double helix, the base “A” (i.e., adenine) pairs with “T” (i.e., thymine). The bases “C” (i.e., cytosine) and “G” (i.e., guanine) pair with one another.
All bases are planar and any hydrogen bonding interactions with the bases are in the plane of the base.
a pentose sugar
Figure 6.3: Different Sugars in Nucleotides
This sugar can either be ribose or deoxyribose. Each numbered carbon on the sugar is followed by a prime mark (i.e., C5’ refers to the fifth carbon of the sugar). A phosphate head is also esterified to the sugar.
Figure 6.4: A and B Forms of Sugars
Sugars can also exist in an A-form or a B-form (i.e., different sugar conformations).
a phosphate
This is linked to the 3’-hydroxyl group the sugar nucleotide via a phosphodiester bond.
Nucleotides are also nitrogenous bases that can either be purines or pyrimidines (observe figure 6.5):
Figure 6.5: Structure of Purines and Pyrimidines
Also observe the manner in which C and N atoms are numbered in purines and in pyrimidines. All purines and pyrimidines also have CO, NH2, and C-CH3 chemical groups!
A nucleoside is the result of coupling a base to a sugar (see figure 6.6 below):
Figure 6.6: A Nucleoside
The base of a nucleoside is linked to the sugar via a N-glycosidic bond. In purines, the attachment is through the N9-C1’ location; the attachment is through the N1-C1’ location for pyrimidines.
Figure 6.7: Base, Nucleoside, and Nucleotide Nomenclature
In summary…
- Base + sugar = nucleoside
- Base + sugar + phosphate = nucleotide
Figure 6.7 also depict the names of several nucleotides and nucleosides.
6.1.1 Chemical Properties of Nucleotides
Figure 6.8: pH of Amino Acids
Nucleotides are strong acids (as observed in figure 6.8).
Figure 6.9: Tautomeric Conversions of Bases
Like figure 6.9 suggests, all bases are also capable of undergoing a conversion to a tautomeric form.
The bases adenine and cytosine are able to isomerize into their imino forms; the bases thymine, guanine, and uracil are capable of isomerizing to their enol forms (however, with a very low frequency of occurrence).
Figure 6.10: Absorption of DNA at Various Wavelengths
All bases and their derivatives absorb light strongly in the near-ultraviolet region of the spectrum (see figure 6.10). This absorption can then be used to determine the nucleic acid concentration via equation (2.1) (i.e., the Beer-Lambert equation).
6.1.2 Stability and Formation of the Phosphodiester Linkage
The synthesis of polynucleotides in vivo involves the hydrolysis of a high-energy nucleoside or deoxynucleoside triphosphate and the formation of a phosphodiester linkage via hydrolysis.
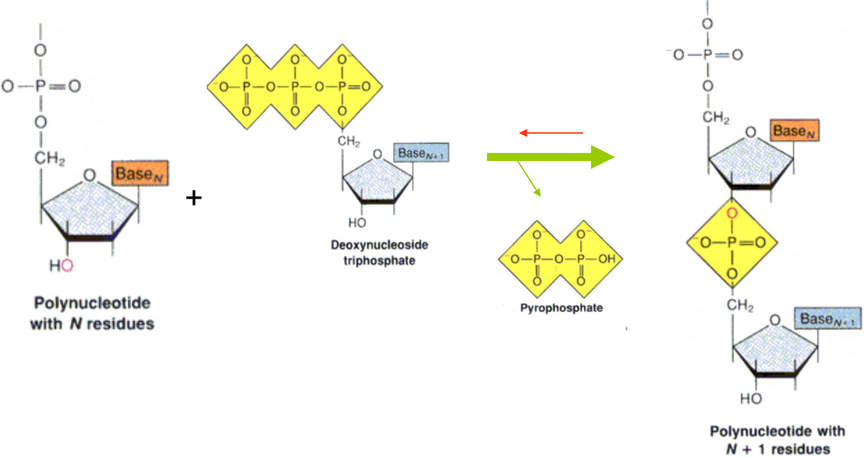
Figure 6.11: Hydrolysis of a Nucleotide
The hydrolysis of triphosphate groups is incredibly energetically favorable. Nucleoside triphosphates (e.g., ATP) are used to provide a driving force for many reactions in vivo.