3.3 Fibrous Proteins
These proteins have a filamentous form and form protein subunits that are capable of polymerizing (i.e., quaternary interactions).
Most fibrous proteins play structural roles in animal cells and tissues, including the skin, the hair, and the connective tissue.
Figure 3.15: Amino Acid Compositions of Fibrous Proteins
Fibrous proteins can be made up of numerous amino acids (see figure 3.15).
Fibrous proteins’ amino acid sequences also favor a particular kind of secondary structure (which determines their mechanical properties).
3.3.1 \(\alpha\)-keratin
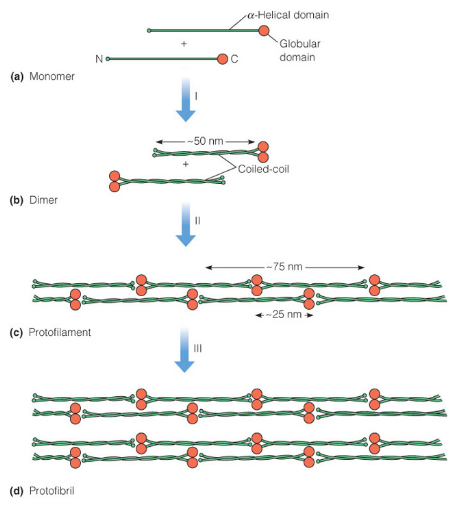
Figure 3.16: Polymerization of Alpha Keratin
This is a fibrous protein that is found in hair, fingernails, claws, horns, and beaks. Its polypeptide consists of over 300 amino acids, is 50 nm long, and has \(\alpha\)-helical rod segments that are capped with globular N- and C-terminal domains (see figure 3.16).
The amino acid sequence of \(\alpha\)-keratin promotes the association of helices to form coiled coils.
The unusually high content of cysteine residues in the primary structure of \(\alpha\)-keratin also allows for extensive cross-linking between chains, hence giving extra strength to the fiber.
3.3.2 Collagen
This is a protein with a very high glycine and proline content (i.e., ~55%). It also has unusual amino acids present - for instance, 4-hydroxyproline (i.e., 4hyP).
Figure 3.17: Left-Handed Helices in Collagen
The primary structure of collagen is especially suitable for forming a triple helix: three intertwined helical strands.
Left-handed helices (see figure 3.17) have 3.3 residues per turn and wrap around one another in a right-handed sense. It is also much more extended than an \(\alpha\)-helix with a rise of 2.9 Angstroms.
The left-handed helix also has long stretches of predominantly Gly-Pro-4hyP. This is because every third residue in the left-handed helix faces the crowded center of the triple helix - only glycine is capable of fitting well.
The amino acids proline and 4hyP help “fix” the geometry of the left-handed helix to stabilize the triple helix.
3.3.3 Fibroin and \(\beta\)-keratin
Figure 3.18: Structure of Fibroin and beta-Keratin
These are proteins produced by insects and spiders; half of these proteins’ primary structures are Gly-Ala repeats (sometimes Gly-Ser).
As seen in figure 3.18, the residues of a \(\beta\)-sheet extend above and below the plane of the sheet, hence placing all glycines on one side and all alanines / serines on another. This allows for strong van der Waals interactions from interdigitation and the close packing of side chains.