1.4 Intermolecular Interactions
The bonds between molecules can be classified into one of three categories depending on their polarities:
- Covalent
- Polar covalent
- Ionic
The aforementioned three categories arise from the different electronegativity values between the atoms of a bond. Electronegativity is a measure of the relative tendency of an atom to attract electrons.
If the difference in electronegativity between two atoms is less than 0.5, then the bond between the two atoms is said to be covalent. Covalent bonds are formed from the sharing of electron pairs between two atoms.
Otherwise, if the difference in electronegativity is between 0.5 and 1.7,1 then the bond is said to be polar covalent.
Figure 1.15: Partial Positive and Negative Charges on a Carbon Monoxide Molecule
When electrons are withdrawn towards oxygen, this is called induction. Induction results in the formation of positive and negative partial charges (i.e., \(\delta+\) and \(\delta-\)).
If the difference in electronegavity between two atoms is greater than 1.7, then the electrons between the two atoms are not shared at all. Rather, the electrons of the bond become solely possessed by the more electronegative atom. This bond is called an ionic bond and is the result of the force of attraction between two oppositely charged ions (i.e., an ionic compound like NaCl).
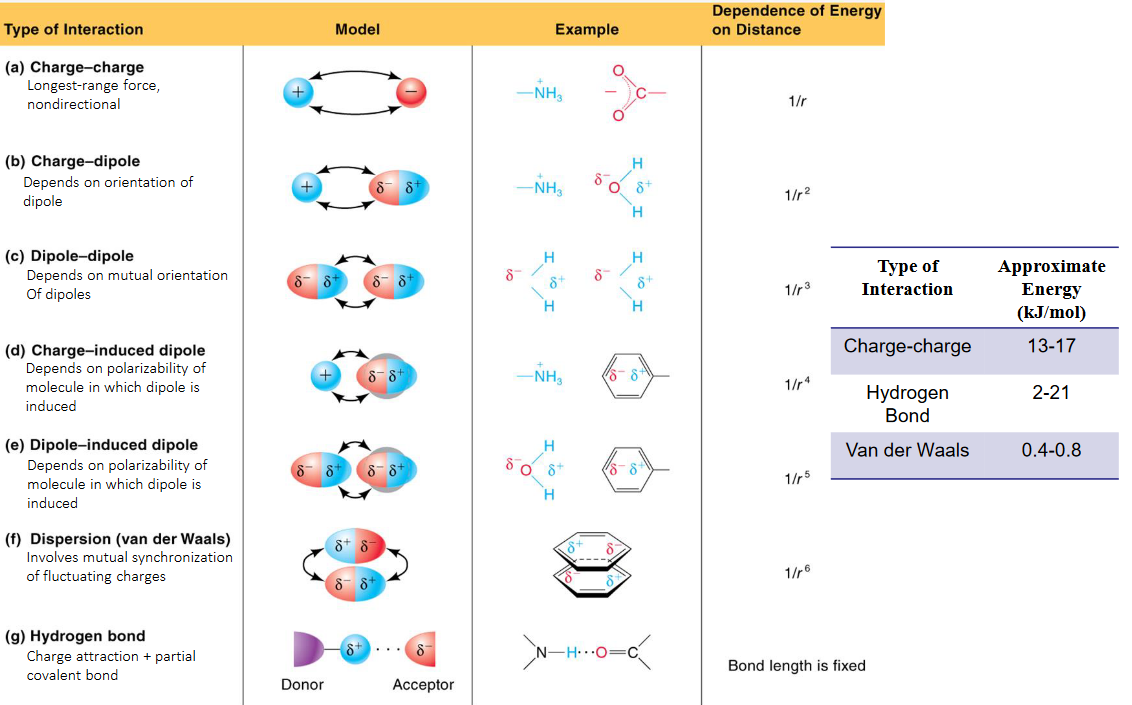
Figure 1.16: Non-covalent Interactions
Noncovalent interactions arise via different mechanisms: they include van der Waals interactions, hydrogen bonding, electrostatic interactions, and also hydrophobic interactions. Figure 1.16 displays numerous noncovalent interactions available.
1.4.1 Hydrophobic interactions
Figure 1.17: Hydrophobic Effect in Action
Many biomolecules: proteins, pigments, certain vitamins, sterols, and phospholipids are amphipathic: they have polar and non-polar groups. The hydrophobic effect (see figure 1.17) is an entropy-based force that “holds” the non-polar regions of biomolecules together.
1.4.2 Ionic interactions
These are the simplest noncovalent interactions between a pair of molecules.
Ionic interactions are also non-directional; biomolecules that exhibit ionic interactions usually also carry a net negative charge.
The predicted force \(F\) between two charged particles is denoted by:
\[\begin{align} F &= \frac{kq_1q_2}{r^2} = \frac{q_1q_2}{4\pi \epsilon_0 r^2} \\ k &= \frac{1}{4\pi \epsilon_0} \end{align}\]
Where:
- \(q_1\) and \(q_2\) are the charges of the particles.
- \(\epsilon_0\) is the vaccum permittivity
- \(r\) is the separation distance
1.4.3 Dipole-dipole movements
Figure 1.18: Examples of Dipole Moments
All polar molecules have a dipole moment that expresses the magnitude of the molecule’s polarity (i.e., how polar is the molecule). Some examples are shown in figure 1.18.
1.4.4 Induced dipoles and van der Waals interactions
Molecules that do not have permanent dipoles can become polar in the presence of an electric field.
Figure 1.19: Induction of a Dipole in Benzene
A molecule is polarizable if and only if a dipole can be induced within it (see figure 1.19 above). The interactions of polarizable molecules are known as induced dipole interactions.
Note that this difference should be viewed as a rough, not an absolute guideline!↩︎