12.4 Fat-Soluble Vitamins
Fat-soluble vitamins are soluble in nonpolar, organic solvents. These vitamins can also be classified into four groups: vitamins A, D, E, and K, all of which contain isoprenoids - compounds with multiple isoprene units and an aromatic ring.
Figure 12.27: Functions of Fat-Soluble Vitamins
While the aforementioned four vitamins have a common structure, each vitamin has a very different function (see figure 12.27)!
12.4.1 Vitamin A
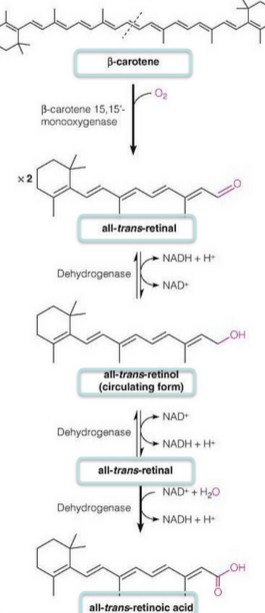
Figure 12.28: Synthesis of Vitamin A from Beta-Carotene
This is also known as retinol (see figure 12.28) - there are three active forms of this vitamin that are collectively referred to as retinoids:
- trans-retinol
- trans-retinal
- trans-retinoic acid
This vitamin can be consumed as an esterified retinol or synthesized from \(\beta\)-carotene - a plant isoprenoid that is especially abundant in carrots.
The oxidation of retinol to retinal is reversible, but the subsequent oxidation to retinoic acid is not!
Nonetheless, vitamin A plays roles in:
- Promoting vision
- Protein synthesis and cell differentiation
- Supporting reproduction and growth
12.4.2 Vitamin D
Vitamin D is a collective name for a group of related lipids.
12.4.2.1 Vitamin D2
This is also known as ergocalciferol (see figure 12.29) and is a commercial product formed via UV irradiation of yeast ergosterol.
Figure 12.29: Structure of Vitamin D2
Vitamin D2 is structurally similar D3 and has the same biological effects as it; vitamin D2 is also added to milk and to butter as a dietary supplement.
12.4.2.2 Vitamin D3
This is also known as cholecalciferol (see figure 12.30) and is nonenzymatically formed in the skin from the steroid 7-dehydrocholesterol.
Figure 12.30: Structure of Vitamin D3
This vitamin is not biologically active; it is converted to 1,25-dihydroxycholecaciferol, a hormone that regulates calcium uptake in the intestines and calcium levels in the bones.
12.4.3 Vitamin E
This is also known as \(\alpha\)-tocopherol (see figure 12.31): a compound with a bicyclic, oxygen-containing ring system with a hydrophobic ring system. The phenol group of vitamin E can undergo oxidation to form a stable free radical.
Figure 12.31: Structure of Vitamin E
Vitamin E is believed to function as a reducing agent that scavenges oxygen and free radicals; while a vitamin E deficiency is rare, it can lead to fragile red blood cells and neurological damage.
Vitamin E deficiencies are commonly caused by genetic defects in the absorption of fat molecules.
12.4.4 Vitamin K
This was originally as a lipid-soluble substance involved in blood coagulation.
Figure 12.32: Structure of Vitamin K1
Phylloquinone (i.e., Vitamin K1 - see figure 12.32) is the plant version of the vitamin that is used in photosynthesis. The role of phylloquinone is completely unrelated to the role of vitamin K in humans.
Figure 12.33: Structure of Vitamin K2
Menaquinone (i.e., Vitamin K2 - see figure 12.33) is the bacterial version of vitamin K. In humans, this can be produced from menadione.
Figure 12.34: Structure of Vitamin K3
Menadione (i.e., Vitamin K3 - see figure 12.34) is a synthetic compound that can be converted into the active forms of vitamin K. While menadione can be readily absorbed, it is found to be toxic - for this reason, it is hardly used in supplements!
12.4.4.1 Vitamin K as a cofactor
Figure 12.35: Vitamin K in an Enzymatic Reaction
Vitamin K is required as an enzyme cofactor in the synthesis of \(\gamma\)-carboxyglutamic acid: a molecule important for the proper functioning of a number of proteins, the most notable of which are clotting factors.
The reduced form of vitamin K serves as a cofactor for the carboxylase that produces the modified glutamate side chain (see figure 12.35).
Note that the formation of the active hydroquinone form of vitamin K and the regeneration of the quinone in vitamin K are all catalyzed by the enzyme vitamin K epoxide reductase (i.e., VKOR).