8 Research design limitations
So far, you have learnt to ask an RQ and design research studies. In this chapter, you will learn to identify limitations to:
- internal validity.
- external validity.
- ecological validity.
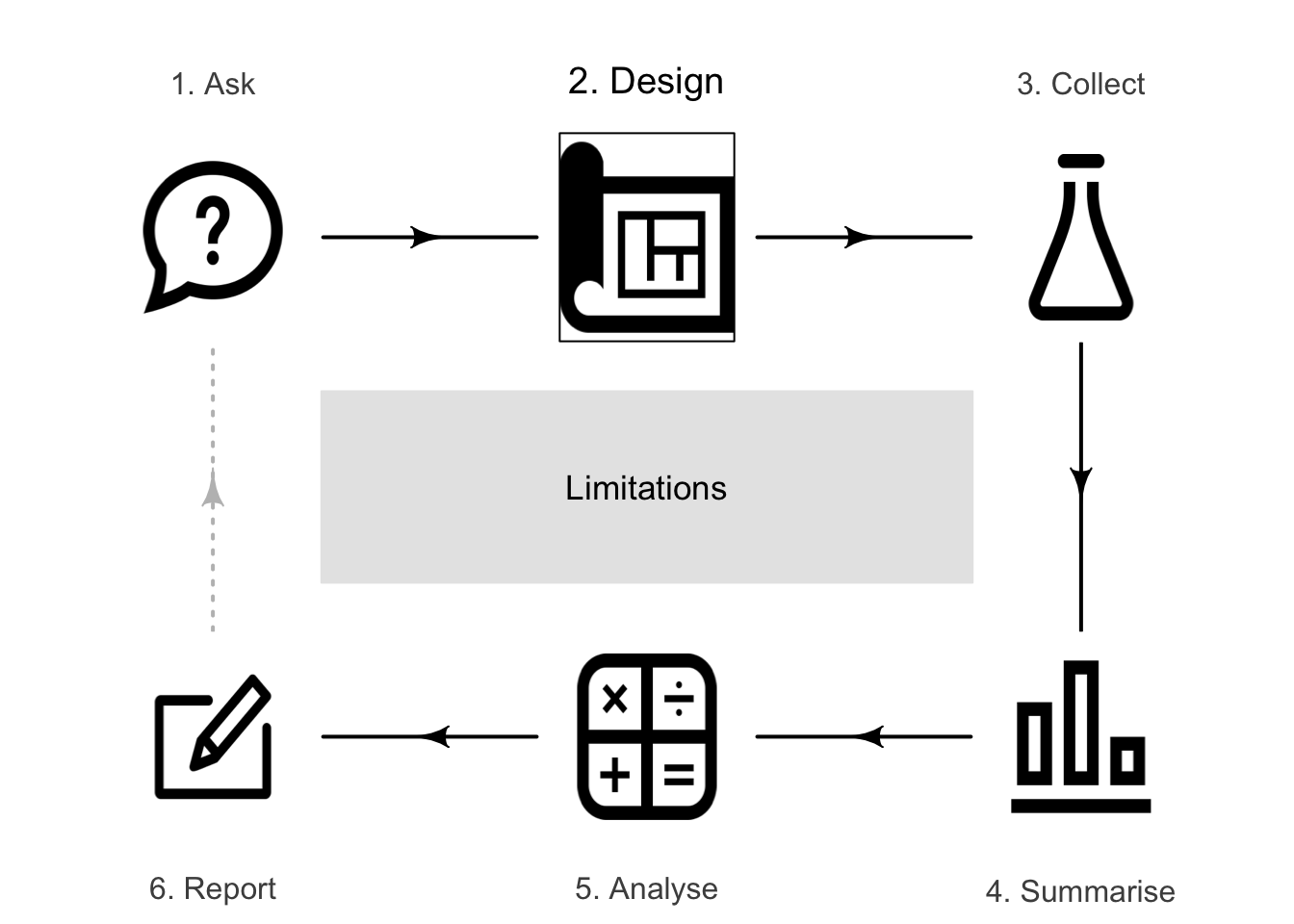
8.1 Introduction
The type of study (Chap. 4) and the research design determine how the results of the study should be interpreted. Ideally, all studies would be perfectly externally and internally valid; in practice this is very difficult to achieve. Practically every study has limitations. The results of a study should be interpreted in light of these limitations. Limitations are not necessarily problems.
Limitations generally can be discussed through three components.
- Internal validity (Sect. 3.1): discuss any limitations to internal validity due to the research design (such as identifying possible confounding variables). This is related to the effectiveness of the study within the sample (Sect. 8.2).
- External validity (Sect. 6.1): discuss how well the sample represents the intended population. This is related to the generalisability of the study to the intended population (Sect. 8.3).
- Ecological validity: discuss how well the study methods, materials and context approximate the real situation of interest. This is related to the practicality of the results to real life (Sect. 8.4).
All these issues should be addressed when considering the study limitations.
Almost every study has limitations. Identifying potential limitations, and discussing the likely impact they have on the interpretation of the study results, is important and ethical.
Different types of research studies have limitations. Experimental studies, in general, have higher internal validity than observational studies, since more of the research design in under the control of the researchers; for example, random allocation of treatments is possible to minimise confounding.
Only well-designed and well-conducted experimental studies can show cause-and-effect relationships.
However, experimental studies may suffer from poor ecological validity; for instance, laboratory experiments are often conducted under controlled temperature and humidity. Many experiments also require that people be told about being in a study (due to ethics), and so internal validity may be comprised (the Hawthorne effect).
Example 8.1 (Limitations due to the study type) Giandomenico, Papineau, and Rivers (2022) studied retro-fitting houses with energy-saving devices, and found large discrepancies in energy savings for observational studies (\(12.2\)%) and experimental studies (\(6.2\)%). The authors say that 'this finding reinforces the importance of using study designs with high internal validity to evaluate program savings' (p. 692).
8.2 Limitations related to internal validity
Internal validity refers to the extent to which a cause-and-effect relationship can be established in a study, eliminating other possible explanations (Sect. 3.1); that is, the effectiveness of the study in the sample. A discussion of the limitations of internal validity should cover, as appropriate: possible confounding and lurking variables; the impact of the Hawthorne, observer, placebo and carryover effects; the impact of any other design decisions.
If anything is likely to compromise internal validity, the implications on the interpretation of the results should be discussed. For example, if the participants were not blinded, this should be clearly stated, and the conclusion should indicate that the individuals in the study may have behaved differently than usual.
Example 8.2 (Study limitations) Axmann et al. (2020) randomly allocated Ugandan farmers to receive, or not receive, hybrid maize seeds. One potential threat to internal validity was that farmers receiving the hybrid seeds could share seeds with their neighbours.
Hence, the researchers contacted the \(75\) farmers allocated to receive the hybrid seeds; none of the contacted farmers reported selling or giving seeds to other farmers. This extra step increased the internal validity of the study.
Maximising internal validity in observational studies is more difficult than in experimental studies (since random allocation is not possible). However, the internal validity of experimental studies involving people is often compromised because people must be informed that they are participating in a study.

Example 8.3 (Internal validity) In a study of the hand-hygiene practices of paramedics (Barr et al. 2017), self-reported hand-hygiene practices were very different from what was reported by peers. That is, how people self-report their behaviours may not align with how they actually behave, which influenced the internal validity of the study.
A study evaluated using a new therapy on elderly men, and listed some limitations of their study:
... the researcher was not blinded and had prior knowledge of the research aims, disease status, and intervention. As such, these could all have influenced data recording [...] The potential of reporting bias and observer bias could be reduced by implementing blinding in future studies.
--- Kabata-Piżuch et al. (2021), p. 10
8.3 Limitations related to external validity
External validity refers to the ability to generalise the findings made from the sample to the entire intended population (Sect. 6.1). For a study to be externally valid, it must first be internally valid: that is, if the study is not effective in the sample studied (i.e., internally valid), the results may not apply to the intended population either.
External validity refers to how well the sample is likely to represent the intended population in the RQ.
If the population is Californians, then the study is externally valid if the sample is representative of Californians. The results do not have to apply to people in the rest of the United States to be externally valid (though this can be commented on too). The intended population is Californians.
External validity depends on how the sample was obtained. Results from random samples (Sect. 6.5) are likely to generalise to the population and be externally valid. (The analyses in this book assume all samples are simple random samples.) Furthermore, results from approximately representative samples (Sect. 6.6) may generalise to the population and be externally valid if those in the study are not obviously different from those not in the study.
Any inclusion criteria, exclusion criteria or control variables may also limit the external validity of the study.
Example 8.4 (External validity) Gammon et al. (2012) identified (for well-documented reasons) a population of interest: 'women of South Asian origin living in New Zealand' (p. 21). The women in the sample were 'women of South Asian origin [...] recruited using a convenience sample method throughout Auckland' (p. 21).
The results may not generalise to the intended population (all women of South Asian origin living in New Zealand) because all the women in the sample came from Auckland, and the sample was not a random sample from this population anyway. The study was still useful however, since we have still learnt information about the population that is represented by the sample, which may be similar to the intended population.
Example 8.5 (Using biochar) Farrar et al. (2018) studied growing ginger using biochar on one farm at Mt Mellum, Australia. The results may only generalise to growing ginger at Mt Mellum, but since ginger is usually grown in similar types of climates and soils, the results may apply to other ginger farms also.
8.4 Limitations related to ecological validity
The likely practicality of the study results in the real world should also be discussed. This is called ecological validity.
Definition 8.1 (Ecological validity) A study is ecologically valid if the study methods, materials and context closely approximate the real situation of interest.
Studies don't need to be ecologically valid to be useful; much can be learnt under special conditions, as long as the potential limitations are understood when applying the results to the real world. The ecological validity of experimental studies may be compromised because the experimental conditions are sometimes highly controlled (for good reason).

Example 8.6 (Ecological validity) Consider a study to determine the proportion of people that buy coffee in a reusable cup. People could be asked about their behaviour. This study may not be ecologically valid, as what people do may not align with what they say they will do (i.e., subjective data).
An alternative study could watch people buy coffee at various coffee shops, and record what people do in practice. (i.e., objective data). This second study is more likely to be ecologically valid, as real-world behaviour is observed.
A study observed the effect of using high-mounted rear brake lights (Kahane and Hertz 1998), which are now commonplace. The American study showed that such lights reduced rear-end collisions by about \(50\)%. However, after making these lights mandatory, rear-end collisions reduced by only \(5\)%. Why?
8.5 Chapter summary
The limitations in a study need to be identified, and may be related to:
- internal validity (effectiveness); how well the study is conducted within the sample, isolating the relationship of interest.
- external validity (generalisability); how well the sample results are likely to apply to the intended population.
- ecological validity (practicality); how well the results may apply to the real-world situation of interest.
8.6 Quick review questions
Are the following statements true or false?
- When interpreting the results of a study, the steps taken to maximise internal validity should be evaluated.
- If studies are not externally valid, then they are not useful.
- When interpreting the results of a study, the steps taken to maximise external validity do not need to be evaluated.
- When interpreting the results of a study, ecological validity is about the impact of the study on the environment.
8.7 Exercises
Answers to odd-numbered exercises are given at the end of the book.
Exercise 8.1 Gentile (2022) examined how people can save energy through lighting choices. The study states (p. 9) that the results 'are limited to the [sample] and cannot be easily projected to other similar settings'.
What type of validity is being discussed here?
Exercise 8.2 Fill the blanks with the correct word: internal, external or ecological.
When interpreting the results of studies, we consider the practicality ( validity), the generalisability ( validity) and the effectiveness ( validity).
Exercise 8.3 A student project asked if 'the average word retention is higher in male students than female students at UniX'. When discussing external validity, the students stated:
We cannot say whether or not the general public have better or worse word retention compared to the students that we will be studying.
Why is the statement not relevant in a discussion of external validity?
Exercise 8.4 Yeh et al. (2018) conducted an experimental study to 'determine if using a parachute prevents death or major traumatic injury when jumping from an aircraft'.
The researchers randomised \(23\) volunteers into one of two groups: wearing a parachute, or wearing an empty backpack. The response variable was a measurement of death or major traumatic injury upon landing. From the study, death or major injury was the same in both groups (\(0\)% for each group). However, the study used 'small stationary aircraft on the ground, suggesting cautious extrapolation to high altitude jumps' (p. 1).
Discuss the internal, external and ecological validity based on this information.
Exercise 8.5 Delaney et al. (2018) examined how well hospital patients sleep at night. The researchers state that 'convenience sampling was used to recruit patients' (p. 2). Later, the researchers state that (p. 7):
... patients requiring hospitalization will likely require some daytime nap periods. This study looks at sleep only in the night-time period \(22\):\(00\)--\(07\):\(00\)h, without the context of daytime sleep considered.
Discuss the internal, external and ecological validity based on this information.
Exercise 8.6 Botelho et al. (2019) examined the food choices made when subjects were asked to shop for ingredients to make a last-minute meal. Half were told to prepare a 'healthy meal', and the other half told just to prepare a 'meal'. The authors emphasise that the purchases were 'simulated' (p. 436):
As participants did not have to pay for their selection, actual choices could be different. Participants may also have not behaved in their usual manner since they were taking part in a research study...
Discuss the internal, external and ecological validity based on this information.
Exercise 8.7 D. Johnson et al. (2018) studied the use of over-the-counter menthol cough-drops in people with a cough. One conclusion from the observational study of \(548\) people was that, taking 'too many cough drops [...] may actually make coughs more severe', as one author explained. Critique this statement.
Exercise 8.8 Suppose a group of students was studying this RQ:
Among Australians, is the average serum cholesterol concentration different for smokers and non-smokers?
The students gave the following information about their study. Explain why each of these statements is incorrect.
- The design is observational, as we cannot manipulate each person's serum cholesterol.
- The outcome is 'the average serum cholesterol concentration for smokers and non-smokers'.
- The study is not externally valid, as the results may not apply to all people in the world.
- The response variable is serum cholesterol.
- In this experiment, the population is 'Australians'.
- The data file will have two columns: one for smokers, and one for non-smokers.
- 'Whether the person owns a cat' is likely to be a confounding variable.
- The observer effect is not relevant, as the participants will know they are involved in a study.
Exercise 8.9 Delarue et al. (2019) discuss studies where subjects rate the taste of new food products. They note that taste-testing studies should be externally and internally valid (p. 78): However, even with good internal and external validity, these studies often result in a 'high rate of failures of new launched products'.
Discuss the internal, external and ecological validity based on this information.