5 Ethics in research
You have learnt how to ask an RQ, and identify different types of studies to obtain data. In this chapter, you will learn to:
- list common ethical issues to be considered in research design.
- understand the purpose of reproducible research.
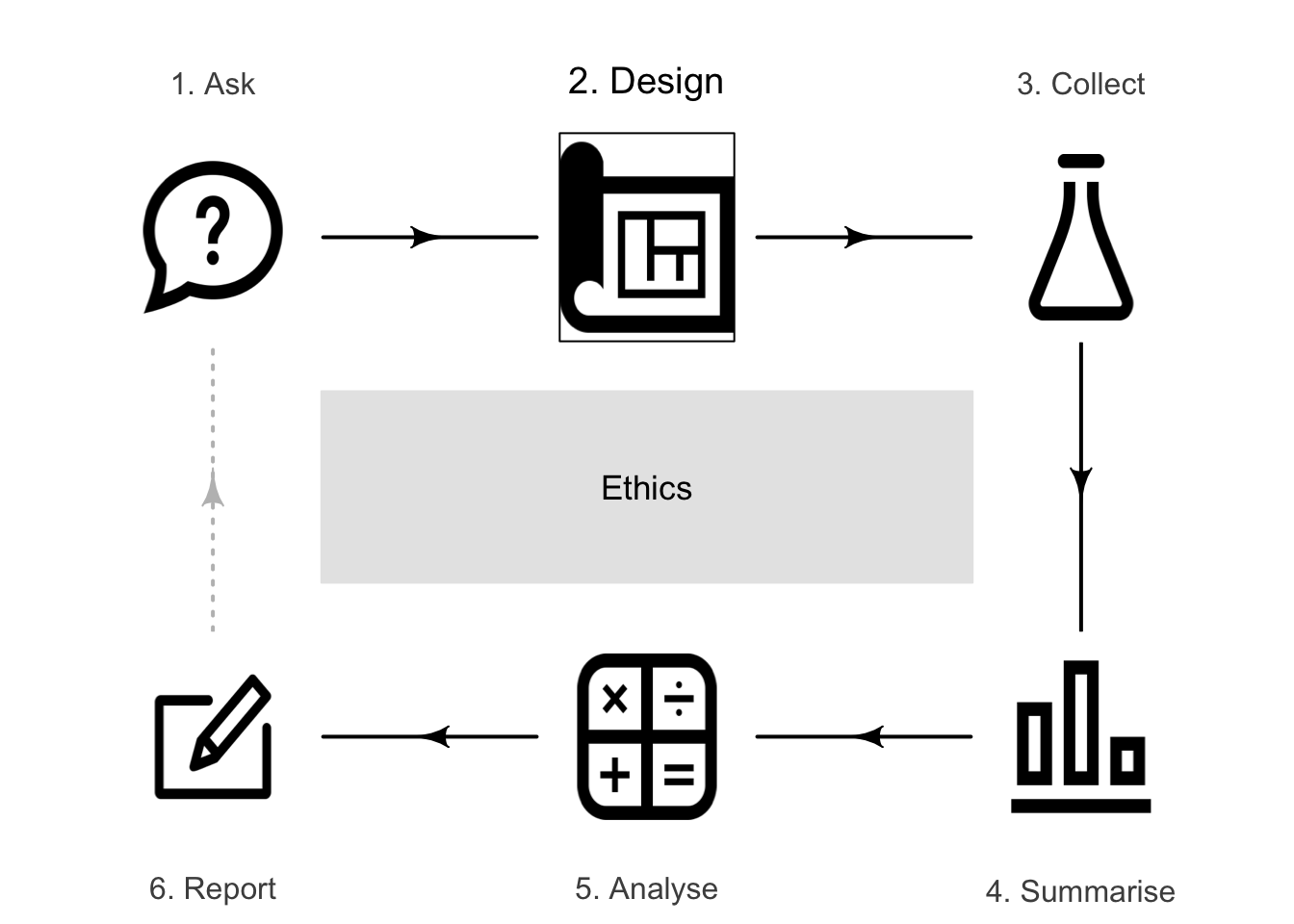
5.1 Introduction: obtaining ethical clearance
All research must be ethical, and must meet ethical guidelines, to minimise risk of harm to the environment, property and to participants, and to preserve the well-being, dignity, rights and safety of participants (including animals). Practically every university and research organisation in the world promotes and enforces ethical research practices.
Most research studies require an ethics committee to formally grant ethics approval before research begins. Only brief comments about research ethics are given here.

Example 5.1 (Ethics) Ethics are important for all studies, not just those involving people or animals. For example:
- in engineering, \(238\) articles published between 1945 and 2015 were retracted, mostly for unethical research practice (Rubbo et al. 2019).
- in the chemical sciences, \(331\) retractions were reported in 2017 and 2018 due to ethical issues, such as falsification of data and plagiarism (Coudert 2019).
5.2 Ethical issues in research design

Ethical issues embrace many areas when designing research studies.
- Acknowledgements: all those who contributed to the research should be acknowledged, including those who prepare figures, take photographs, or have helped collect data.
- Analysis: the analysis must use appropriate methods.
- Confidentiality: data should be kept confidential and secure.
- Consent: when appropriate, people should consent to being in the study, and hence should be told what the study involves. People should also be able to withdraw from the study without penalty.
- Economic risks: financial loss to participants should be avoided. Reimbursements of reasonable costs for participating may be appropriate.
- Environmental risks: environmental impacts and damage should be avoided or minimised.
- Funding: sources of funding should be disclosed. Any studies funded by, or sanctioned by, companies or organisations with vested interests need to be carefully scrutinised. These may lead to, or may give the impression of, conflicts of interest.
- Incentives to participate: if participants are offered incentives to participate (above reimbursement of costs), these should be acknowledged as it may cause (perhaps unconsciously), or may give the impression of causing, participants to influence the results.
- Legal risks: participants should not be put in the position of breaking laws, and the research itself should not break any laws.
- Plagiarism: the work of others should be appropriately acknowledged and not claimed to be original (see Sect. 35.5.7).
- Physical risks: physical harm or discomfort (to researchers, participants or bystanders) should be avoided or minimised.
- Psychological risks: psychological harm or discomfort (to researchers, participants or bystanders) should be avoided or minimised.
- Resourcing: the study should not waste resources, time or money (e.g., if the answer to the RQ is already known, the study is not necessary).
- Sample size: the study should not use more individuals than necessary.
- Social risks: social harm or discomfort (to researchers, participants or bystanders) should be avoided or minimised.
- Storage of data: data should be stored securely, kept for the required amount of time, then (if appropriate) securely disposed.
Example 5.2 (Poor ethics) In the Tuskegee syphilis experiment, conducted between 1932 and 1972, treatments were actively withheld from men with syphilis (Corbie-Smith 1999). The men's wives and children were often affected, and the men were lied to about their treatments. This study was highly unethical, and could not be conducted now.
Example 5.3 (Poor ethics in analysis) In 1986, the American space shuttle Challenger exploded just after launch, killing all seven astronauts on board. A review (Dala, Fowlkes, and Hoadley 1989) found the cause was partly that engineers failed to use some data that should have been used. This was unethical.
5.3 Reproducible research
One way to ensure that research results are reliable and trustworthy is through reproducible research: enabling someone else to repeat the study and analysis, to confirm the findings. For research to be reproducible, the methods, data, analysis methods and relevant computer code must be available (Laine et al. 2007) when possible. (Sometimes releasing data is unethical, such as when individuals may be identified, so should not be released.)
Methods for ensuring reproducible research are often discipline dependent, and beyond the scope of this book. Different journals also have different expectations regarding reproducibility. Nonetheless, the basic ideas are important.
The importance of reproducibility in the analysis phase is crucial; for example:
There are serious medical consequences to errors attributable to the effects of spreadsheet programs and software operated through a graphical user interface [...] that could have been avoided through a reproducible research paradigm...
--- Simons and Holmes (2019), p. 471
Using purely point-and-click interfaces for statistical analysis (e.g., spreadsheets) is not recommended, as results are not reproducible.
Rather than using spreadsheets (see Sect. 2.12), using tools which encourage reproducible research are recommended. Statistical software packages, such as jamovi, Python, R, SAS, SPSS and Stata, are recommended as the analysis commands can be recorded (even when using the point-and-click interfaces), and hence the analysis is reproducible.
5.4 Chapter summary
Studies must be ethical, and any formal study must obtain ethical approval before beginning. Ethics covers issues including, but not restricted to:
- acknowledgements.
- analysis methods.
- confidentiality.
- consent.
- economic risks.
- environmental risks.
- funding.
- incentives for participants.
- legal risks.
- plagiarism.
- physical risks.
- psychological risks.
- resourcing.
- sample size.
- social risks.
- storage of data.
5.5 Quick review questions
Are the following statements true or false?
- Ethics apply for any type of study.
- Ethics only refer to the interactions of the researchers with participants in the study.
- Ethics only apply when people are the individuals.
- Ethics only apply when people or animals are the individuals.
- Ethics can extend to storage of data.
- Ethics only apply to the design of the study.
- Ethics apply even to the analysis of the data.
5.6 Exercises
Answers to odd-numbered exercises are given at the end of the book.
Exercise 5.1 Consider this conundrum (Crozier and Schulte-Hostedde 2015):
A research team has an extraordinarily successful long-term study of a population of bighorn sheep (Ovis canadensis) on Ram Mountain...
The population contains marked individuals for which the research team has incredibly detailed data [...] this research has lead to numerous important publications.
Recently, however, a cougar (Puma concolor) that has learned to specialize on these sheep is slowly but surely eating all of them. This is a study of a natural population, which includes predation, but this cougar is drastically reducing the sample size of the study.
Since it is legal to hunt cougars in the region where this study is taking place, one option is to try to kill the predator; however, even if a cougar were successfully hunted, this would not ensure that it was the correct one.
What action would you recommend, from an ethical point-of-view?
Exercise 5.2 Suppose a research group is testing a new drug, with the potential to cure a debilitating illness. The researchers could (a) use a control group that does not receive the new drug, and so obtain stronger evidence for using the drug if it works; or (b) not use a control group, so that everyone in the study potentially benefits from the using the new drug.
What would you decide? Explain.
Exercise 5.3 Suppose a very deadly and highly contagious disease breaks out. Is it ethical to use a new drug to treat those affected, even though the drug is experimental and the potentially harmful side effects are unknown? Discuss your point-of-view.
Exercise 5.4 Is it ethical to lie to subjects? Deception is used in some disciplines, and may be approved by ethics committees under some circumstances (such as potential benefits of the study, and whether the deception may cause physical or psychological discomfort to the participants).
Is it ethical to tell participants that they are taking an active medication, when it is actually ineffective (a 'placebo')? Discuss the advantages and disadvantages.