Chapter 3 Clinical measurement and agreement
This chapter explores the potential bias and precision of clinical measurements, addressing both repeated measurements taken by the same observer and those taken by different observers. It introduces key concepts such as reliability, repeatability, and agreement.
3.1 Precision of clinical measurements
Significant figures inform about the precision with which measurements are being reported. They consist of all non-zero digits, zeros between non-zero digits, and zeros following the last non-zero after the decimal point. Significant figures are not the same as decimal places, which are all the digits after the decimal point. The difference between the two concepts is illustrated in Table 3.1. Observations should be rounded to a few significant figures only for reporting, after all the calculations have been done.
| Reported measurement | Number of significant figures | Number of decimal places |
|---|---|---|
| 1.5 | 2 | 1 |
| 0.15 | 2 | 2 |
| 1.66 | 3 | 2 |
| 0.0011 | 2 | 4 |
| 0.00110 | 3 | 5 |
3.1.1 Measurement error
Multiple measurements of the same quantity on the same subject will typically differ due to measurement error. To estimate the size of measurement error, the within-subject standard deviation, \(s_w\), can be calculated. To do so, a set of replicate readings is needed, where each subject in the sample is measured more than once. The within-subject standard deviation, \(s_w\), can then be calculated using one-way analysis of variance (ANOVA).
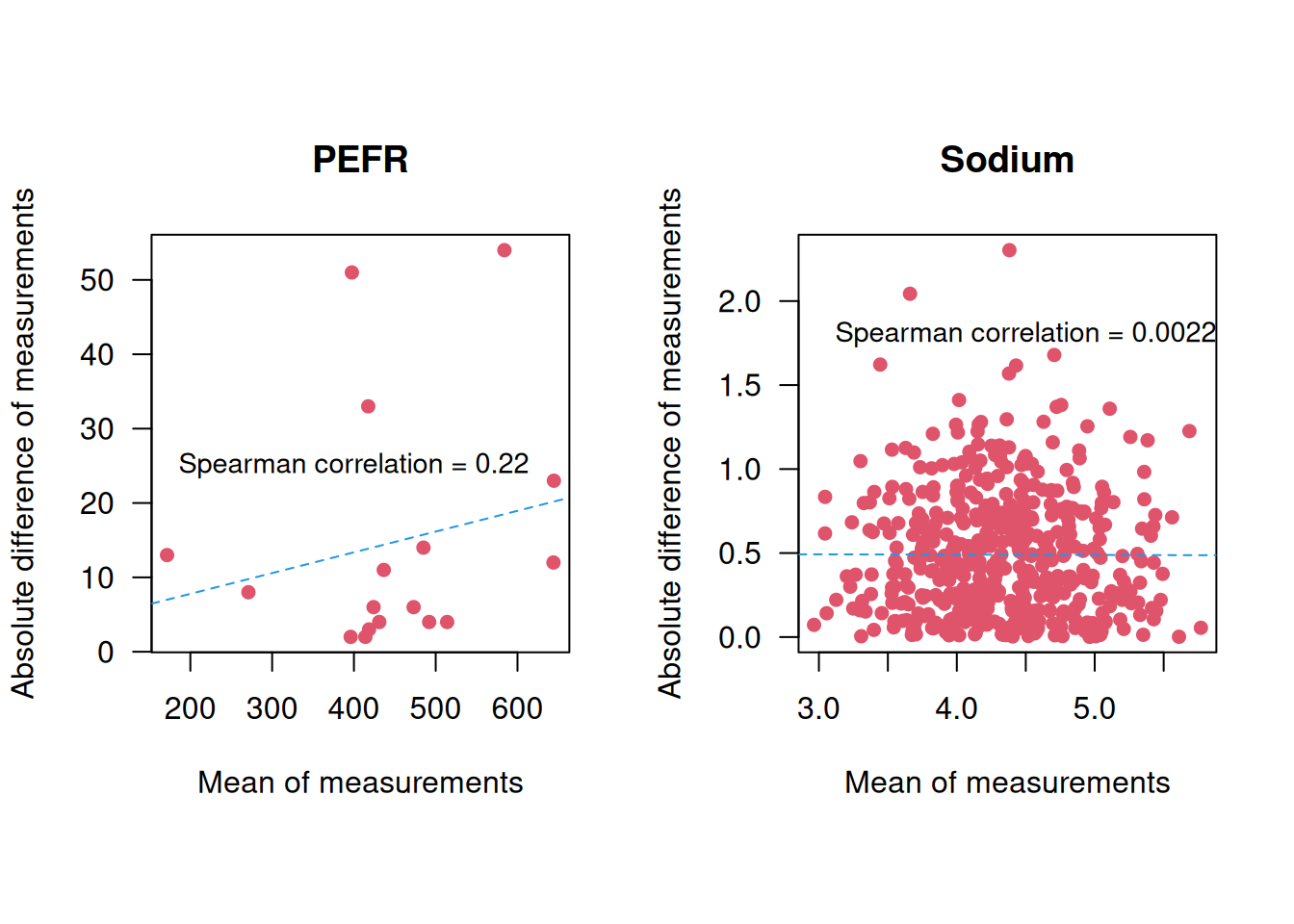
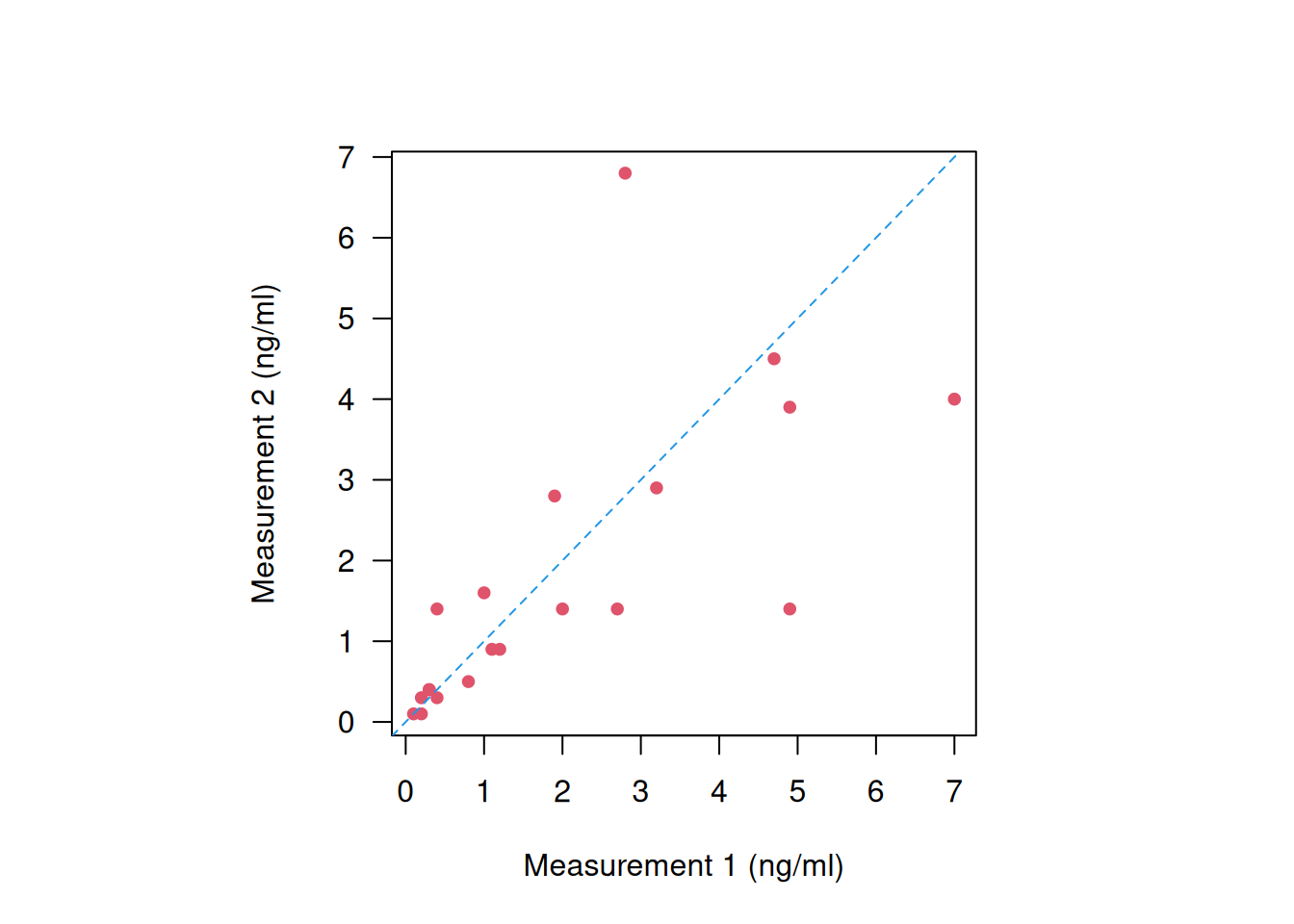
Example 3.1 A peak flow meter is a device to measure the maximal expiration speed of a person (faster = “better”). The Peak Expiratory Flow Rate (PEFR) data from Bland (2015) (Chapter 20.2) contains 17 pairs of readings made by the same observer with a Wright Peak Flow Meter (in litres/min) on 17 healthy volunteers, see Figure 3.1.
## Subject First Second
## 1 1 494 490
## 2 2 395 397
## 3 3 516 512
## 4 4 434 401
## 5 5 476 470
## 6 6 557 611
## 7 7 413 415
## 8 8 442 431
## 9 9 650 638
## 10 10 433 429
## 11 11 417 420
## 12 12 656 633![Repeated measurements of peak expiratory flow rate [@bland].](bookdown-demo_files/figure-html/PEFR-1.png)
Figure 3.1: Repeated measurements of peak expiratory flow rate (Bland, 2015).
Example 3.2 The TONE study is a randomized controlled trial aimed at determining whether a reduction in sodium intake can effectively control blood pressure (Appel et al, 1995). Nab et al (2021) simulated a dataset based on the TONE study, containing for each participant: the sodium intake in mg measured by a 24h recall, a binary variable indicating whether the participant was assigned to a usual or sodium-lowering diet, and two measurements of sodium intake in mg measured in urine. We focus here on the participants for which the two urinary measurements are available. The two sodium intake measurements are displayed in Figure 3.2.
![Repeated measurements of urinary sodium intake [@Nab2021].](bookdown-demo_files/figure-html/sodium-1.png)
Figure 3.2: Repeated measurements of urinary sodium intake (Nab et al, 2021).
The one-way ANOVA for the PEFR date (Example 3.1) is computed as follows:
## [1] 17## Df Sum Sq Mean Sq F value Pr(>F)
## subject 16 441599 27600 117.8 3.15e-14 ***
## Residuals 17 3983 234
## ---
## Signif. codes:
## 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1The entry Residuals Mean Sq gives the within-subject variance
\(s_w^2 = 234\), so the within-subject standard deviation is
\(s_w = \sqrt{234} = {15.3}\) litres/min.
For the sodium data (Example 3.2),
the within-subject standard deviation is calculated with
the following Rcode:
measurement_sod <- c(sodium_f$urinary1, sodium_f$urinary2)
subject_sod <- rep(1:nrow(sodium_f), times = 2)
aov_sod <- aov(measurement_sod ~ subject_sod)
(sigmaw_sod <- sqrt(summary(aov_sod)[[1]][2,3]))## [1] 0.6128869and so \(s_w = 0.6\) mg.
It is also possible to calculate a confidence interval for the within-subject
standard deviation \(s_w\) using the following R function:
confintSD <- function(sd, dof, level = 0.95){
# sd: standard deviation
# dof: degree of freedom
# level: confidence level
alpha <- 1 - level
p_lower <- alpha/2
p_upper <- 1-alpha/2
c(lower = sqrt(dof / qchisq(p_upper, df = dof)) * sd,
estimate = sd,
upper = sqrt(dof / qchisq(p_lower, df = dof)) * sd)
}
# for PEFR data
round(confintSD(sd = sigmaw, dof = 17), 1) ## lower estimate upper
## 11.5 15.3 22.9## lower estimate upper
## 0.58 0.61 0.65Derivation details are given in Appendix A.5.
A simpler way to compute \(s^2_w\) (which gives approximately the same result)
is based on the variance \(s^2_d\) of the
differences Diff between the two measurements,
\(s^2_w = s^2_d/2\) (assuming independence between groups).
For the PEFR data (Example 3.1), this
gives
## compute the differences
PEFR$Diff <- PEFR[, "First"]-PEFR[, "Second"]
## variance of the differences
varDiff <- var(PEFR$Diff)
## within-subject variance is half the variance of the differences
varWithin <- varDiff/2
sw <- sqrt(varWithin)
print(round(sw, 1))## [1] 15.4However, this approach cannot be used if there are more than two measurements for some subjects.
3.1.3 Repeatability
Definition 3.1 Repeatability coefficient is the value below which the difference \(d\) between two measurements will lie with a certain probability, usually 0.95.
Repeatability is usually computed as \(1.96 \sqrt{2} s_w \approx 2 \sqrt{2} s_w \approx 2.8 s_w\), where \(s_w\) is the within-subjects standard deviation. Note that the standard deviation of \(d\) is \(s_d = \sqrt{2} s_w\). The repeatability is \(43.3\) litres/min in the PERF data (Example 3.1) with 95% CI
## lower estimate upper
## 32.5 43.3 64.9and \(1.7\)mg in the sodium data (Example 3.2) with 95% CI
## lower estimate upper
## 1.3 1.7 2.6The limits of the confidence interval for \(2\sqrt{2}s_w\) are calculated by multiplying the limits of the confidence interval for \(s_w\) by \(2\sqrt{2}\).
The value \(2 \sqrt{2} s_w\) is also called the smallest real difference, because it is the smallest difference that can be interpreted as evidence for a real change in the subject’s true value.
3.1.4 Reliability and the intraclass correlation coefficient
Reliability of a measurement is characterized by the correlation coefficient between pairs of readings.
Pearson correlation coefficient between the first and second measurement depends on the ordering of pairs, and is therefore not appropriate to assess the reliability between two pairs of measurements. To illustrate this problem, we change the ordering of some random pairs in the PERF data:
## [1] 1 2 4 7 11 13 14 16## First Second First.new Second.new
## [1,] 494 490 490 494
## [2,] 395 397 397 395
## [3,] 516 512 516 512
## [4,] 434 401 401 434
## [5,] 476 470 476 470
## [6,] 557 611 557 611## [1] 0.9834307## [1] 0.9823037In contrast, the intraclass correlation coefficient (ICC) does not depend on the ordering of the pairs and should be used instead. The ICC is the average correlation among all possible ordering of pairs, and extends easily to the case of more than two observations per subject. It can be defined based on the between- and within-subject variances:
Definition 3.2 The Intraclass Correlation Coefficient (ICC) relates the between-subject variance \(s_b^2\) to the total variance \(s_b^2 + s_w^2\) of the measurements: \[\begin{equation*} \mathrm{ICC} = \frac{s_b^2}{s_b^2 + s_w^2}. \end{equation*}\]
The between-subject variance \(s^2_b\) can also be read-off from an ANOVA,
\(s^2_b =\) (subject Mean Sq \(-\) Residuals Mean Sq)\(/2\).
A convenient way to compute ICC is to use the function ICC::ICCbare():
## [1] 0.983165The ICC package also provides a function to calculate confidence intervals
for the ICC:
sel <- c("LowerCI", "ICC", "UpperCI")
## Compute CIs
ICC.CI <- ICCest(x=subject, y=measurement)[sel]
print(t(ICC.CI))## LowerCI ICC UpperCI
## [1,] 0.9552393 0.983165 0.9938183ICC can be interpreted as the proportion of the variance of measurements which is due to the variation between true values rather than to measurement error. Therefore, ICC depends on the variation between subjects and thus relates to the population from which the sample was taken. This method should hence be used only when we have a representative sample of the subjects of interest (Bland, 2015).
Example 3.1 (continued) For illustration we modify the PERF data to have less variation between subjects, but the same within-subject standard deviation, see Figure 3.6.
mymeans <- apply(cbind(First, Second), 1, mean)
shrinkage <- 0.5*(mymeans-mean(mymeans))
ShrunkenFirst <- First - shrinkage
ShrunkenSecond <- Second - shrinkage
ShrunkenMeasurement <- c(ShrunkenFirst, ShrunkenSecond)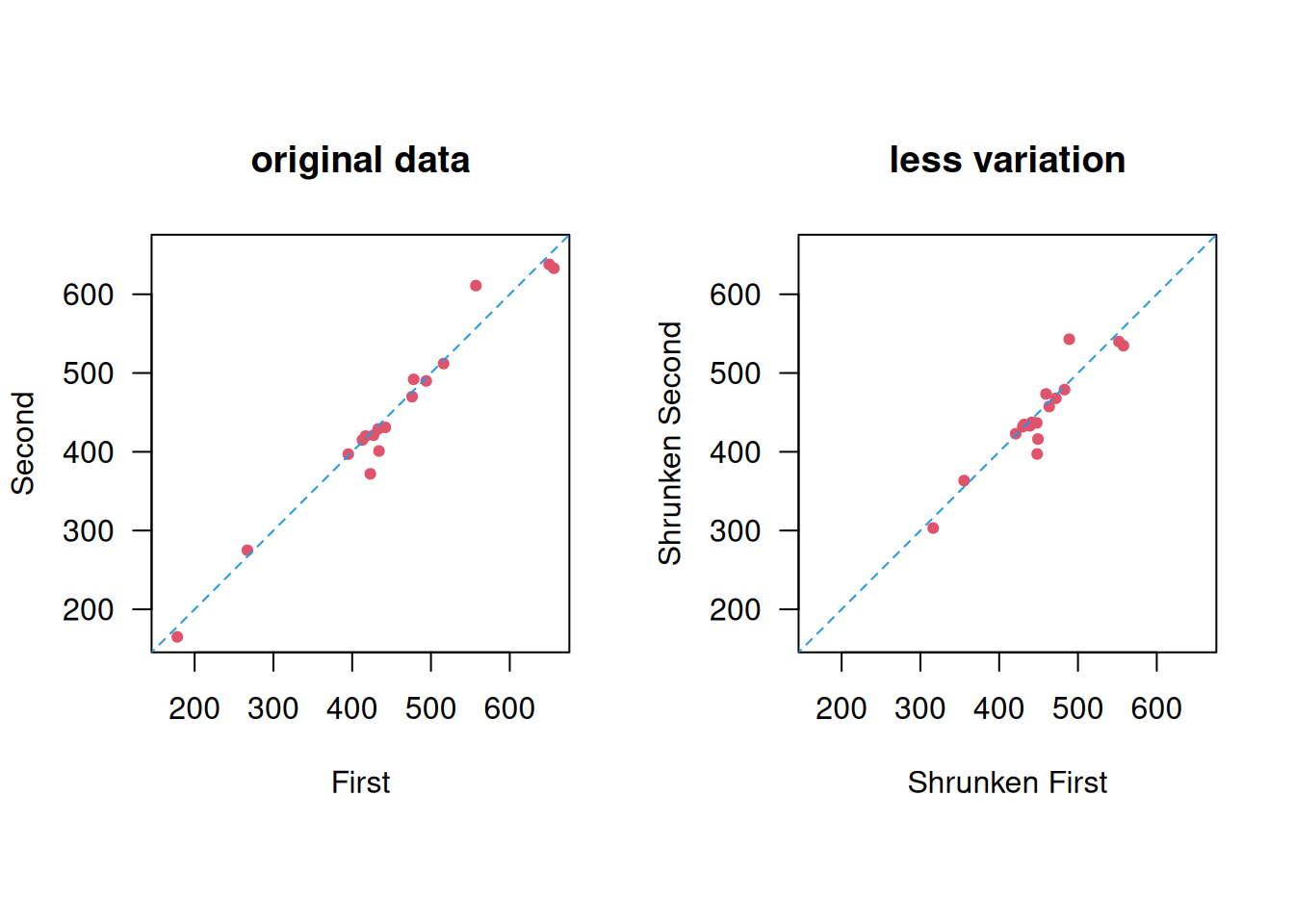
Figure 3.6: Less between-subject variation.
## Df Sum Sq Mean Sq F value Pr(>F)
## subject 16 110400 6900 29.45 2.79e-09 ***
## Residuals 17 3983 234
## ---
## Signif. codes:
## 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1The within-subject standard deviation doesn’t change: \(s_w = \sqrt{234} = {15.3}\) litres/min, but the ICC is smaller:
## [1] 0.93431873.2 Agreement of two methods with continuous measurements
In Section 3.1, we discussed repeatability and reliability, focusing on how well replicates from the same observer align. Now, the focus shifts to examining the agreement between different observers (subjects, machines, methods, etc.).
Example 3.4 A total of \(n=39\) pairs of blood glucose measurements (in mmol/L) were measured with two different devices: the Cobas6000 and the Hitachi912. Figure 3.7 displays the results from a correlation and a regression analysis to assess the agreement between the two devices. However, neither of these methods is useful to assess agreement.
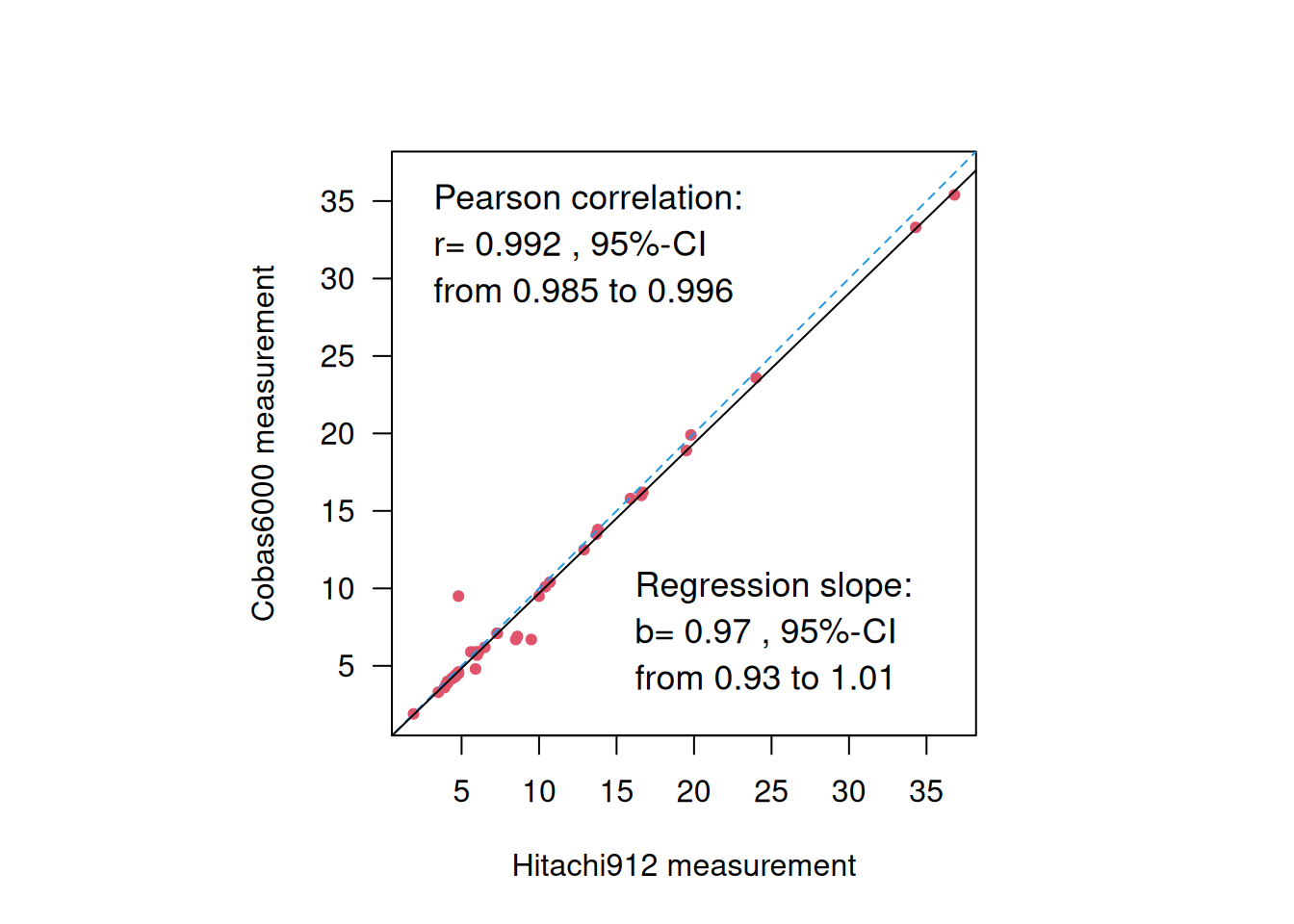
Figure 3.7: Blood glucose measurements from two different devices.
First, correlation is not useful to assess agreement, because multiplication of measurements from one device with any factor will not change the correlation, so devices that do not agree may show high correlation. For example, the correlation between 10 \(\times\) Cobas6000 and Hitachi912 measurements remains the same:
##
## Pearson's product-moment correlation
##
## data: 10 * co and hi
## t = 48.211, df = 37, p-value < 2.2e-16
## alternative hypothesis: true correlation is not equal to 0
## 95 percent confidence interval:
## 0.9849374 0.9958996
## sample estimates:
## cor
## 0.9921343Furthermore, a regression slope \(b \approx 1\) does also not necessarily mean good agreement, because adding a constant to the measurements from one device will not change the regression slope, so two methods that do not agree may still have a slope \(\approx\) 1. For example the regression slope for 10 + Cobas6000 vs. Hitachi912 measurements remains around 1:
| Coefficient | 95%-confidence interval | \(p\)-value | |
|---|---|---|---|
| Intercept | 10.00 | from 9.48 to 10.52 | < 0.0001 |
| hi | 0.97 | from 0.93 to 1.01 | < 0.0001 |
3.3 The method of Bland & Altman
The method of Bland & Altman was introduced in 1986, see the original publication in Figure 3.8. It extends the concept of repeatability introduced in Section 3.1.3 to observations from different devices to introduce the limits of agreement. The original publication is one of the most cited papers in medical research with more than 50’000 citations by the end of 2023.

Figure 3.8: The original publication by Bland & Altman.
The Bland-Altman plot assesses the dependency of measurement error (size and variability) on the “true” value. The \(x\)-axis gives the mean \((x_i + y_i)/2\) of both measurements as an estimate of true value. The \(y\)-axis gives the difference \(d_i = x_i - y_i\) of both measurements to eliminate variability between observations. The bias, i.e. the mean difference \(\bar{d}\) (systematic error) with its confidence interval, and the limits of agreement (LoA) can be read off the Bland-Altman plot. Approximately 95% of points will lie between
\[\underbrace{\bar{d} - 2 \cdot s_d}_{\mbox{LoA}_{\tiny \mbox{low}}} \mbox{ and } \underbrace{\bar{d} + 2 \cdot s_d}_{\mbox{LoA}_{\tiny \mbox{up}}}.\]
Here \(s_d=\sqrt{2} s_w\) is the standard deviation of the differences. If differences within the limits of agreement are considered not clinically relevant, then the two methods are considered exchangeable.
3.3.1 Confidence intervals for limits of agreement
Under normality, \(\bar{d}\) and \(s_d\) are independent. The variance of \(\bar d\) is estimated by \(s_d^2/n\), with \(n\) the sample size. The variance of \(s_d\) can be approximately estimated by \(s_d^2/2n\) [see Bland and Altman (1986); Bland and Altman (1999); Appendix A.5]. Hence, the standard error of the limits of agreement is estimated by \[\begin{eqnarray*} \mbox{se}(\mbox{LoA}_{\tiny \mbox{low}}) &= \mbox{se}(\mbox{LoA}_{\tiny \mbox{up}}) \\ &= \mbox{se}(\bar{d} \pm 2 \cdot s_d) \\ &= \sqrt{\mathop{\mathrm{Var}}(\bar{d} \pm 2 \cdot s_d)} \\ &= \sqrt{\mathop{\mathrm{Var}}(\bar d) + 4 \mathop{\mathrm{Var}}(s_d)} \\ & \approx \sqrt{s_d^2/n + 4 s_d^2/2n} \\ &= \sqrt{3/n} \, s_d \ . \end{eqnarray*}\] This can be used to compute 95% CI for \(\mbox{LoA}_{\tiny \mbox{low}}\) and \(\mbox{LoA}_{\tiny \mbox{up}}\). The standard error can also be used to compute the required sample size \(n\) for a fixed width \(w\) of the 95% confidence interval for the limits of agreement. A rule of thumb by Martin Bland is: “I usually recommend \(n\)=100 as a good sample size, which gives a 95% CI about \(\pm\) 0.34 \(s_d\).”
Example 3.4 (continued) The Bland-Altman plot for the glucose measurements is shown in Figure 3.9. The bias is 0.32 mmol/L with 95% CI from -0.01 to 0.65 (left). The limits of agreement are \(\mbox{LoA}_{\tiny \mbox{low}}\) = -1.67 mmol/L with 95% CI from -2.24 to -1.10 and \(\mbox{LoA}_{\tiny \mbox{up}}\) = 2.30 mmol/L with 95% CI from 1.74 to 2.87 (right).
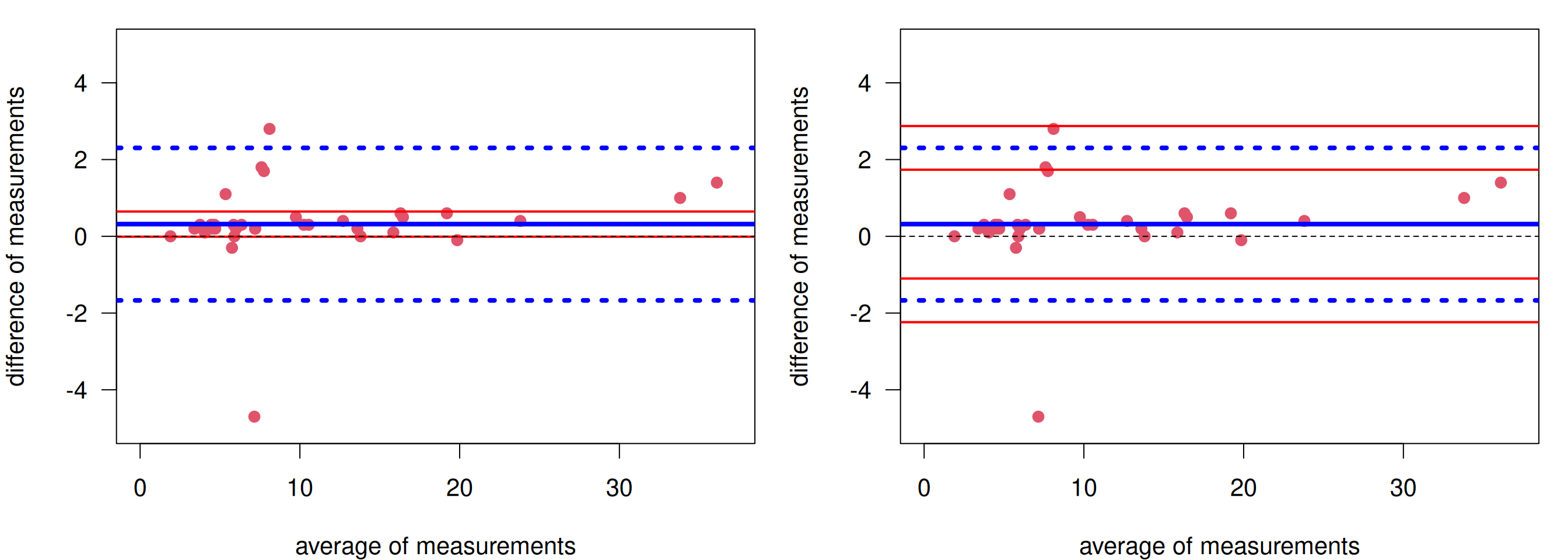
Figure 3.9: Bland-Altman plot for glucose measurements. Left: With confidence interval for the bias. Right: With confidence intervals for the limits of agreement.
In order to know whether Hitachi912 can be replaced with Cobas6000, one has to determine a priori what difference \(\delta\) is clinically relevant. If the limits of agreement (lower = -1.67, upper = 2.30) (or the corresponding confidence intervals) are within \([-\delta, \delta]\), then the old device can be replaced.
3.4 Agreement of two methods with binary variables
Example 3.5 In a study of chest radiography in children in Pakistan (see Hazir et al (2006)), children aged 2 to 59 months with non-severe pneumonia were invited to participate. In total 2000 children were enrolled, for whom 1848 chest radiographs were available for assessment. Two consultant radiologists used standardized criteria to evaluate the chest radiographs, with no clinical information available to them. The primary outcome was diagnosis of pneumonia (absent or present) from chest radiographs.
| Radiologist A | Pneumonia | No pneumonia | Total |
|---|---|---|---|
| Pneumonia | 176 | 240 | 416 |
| No pneumonia | 83 | 1349 | 1432 |
| Total | 259 | 1589 | 1848 |
The proportion of agreement between the two classifications is \[\begin{eqnarray*} p_\mathrm{obs}&=& (1349 + 176) / 1848 \ = \ 0.83. \end{eqnarray*}\] The problem is that two “random” classifiers would also agree for some patients, so we need to adjust for this agreement by chance. We can calculate this based on the expected frequencies under independence. For example, the expected frequency in the first cell of Table 3.2 is \(259 \cdot 416 / 1848 = 58.3\).
| Radiologist A | Pneumonia | No pneumonia | Total |
|---|---|---|---|
| Pneumonia | 58.3 | 200.7 | 416 |
| No pneumonia | 357.7 | 1231.3 | 1432 |
| Total | 259 | 1589 | 1848 |
Table 3.3 gives all four expected frequencies. The expected proportion of agreement by chance then is \[\begin{eqnarray*} p_\mathrm{exp}%&=& (n_{1+} n_{+1} + n_{2 +} n_{+ 2})/n_{++}^2 \\ &=& (1231.3 + 58.3) / 1848 \\ &=& 0.70. \end{eqnarray*}\]
3.4.1 Cohen’s Kappa
Definition 3.3 Cohen’s Kappa is the proportion of agreement beyond chance \(p_\mathrm{obs}- p_\mathrm{exp}\), standardized with the maximal possible value \(1-p_\mathrm{exp}\):
\[\begin{eqnarray*} \kappa &=& \frac{p_\mathrm{obs}- p_\mathrm{exp}}{1 - p_\mathrm{exp}} \end{eqnarray*}\]
It has the following properties: \(\kappa \leq p_\mathrm{obs}\), \(\kappa = 1\) means that there is perfect agreement (\(p_\mathrm{obs}=1\)), \(\kappa = 0\) means that the agreement not larger than expected by chance (\(p_\mathrm{obs}=p_\mathrm{exp}\)), and the smallest possible value of \(\kappa\) is \(-p_\mathrm{exp}/(1-p_\mathrm{exp})\). A negative \(\kappa\) means that the agreement is worse than by chance.
In Example 3.5 we obtain
\[\begin{eqnarray*} \kappa &=& \frac{0.83- 0.70}{1 - 0.70} = 0.42. \end{eqnarray*}\]
A classification of Cohen’s Kappa from Altman (1991) and Bland (2015) is given in Table 3.4.
| Value of kappa | Strength of agreement |
|---|---|
| \(0.00 - 0.20\) | Poor |
| \(0.21 - 0.40\) | Fair |
| \(0.41 - 0.60\) | Moderate |
| \(0.61 - 0.80\) | Good |
| \(0.81 - 1.00\) | Very good |
However, there is a caveat with the interpretation shown in Table 3.4: \(\kappa\) also depends on the true proportion of subjects in the two categories. Figure 3.10 shows the expected \(\kappa\) as a function of the true proportion of “Yes” subjects for different probabilities of agreement. We can see that expected \(\kappa\) drops for extreme proportions close to zero or one.
In Table 3.5, \(\kappa = 0.42\) although \(p_\mathrm{obs}= 1525\)/\(1848\) = \(0.83\). The problem is that the observed proportion of “positive” subjects is very low (5/94 resp. 6/94). This shows that \(\kappa\) also depends on the marginal distribution of positive (respectively negative) subjects!
Assume the two observers have the same chance of a correct assessment, we have the following figure.
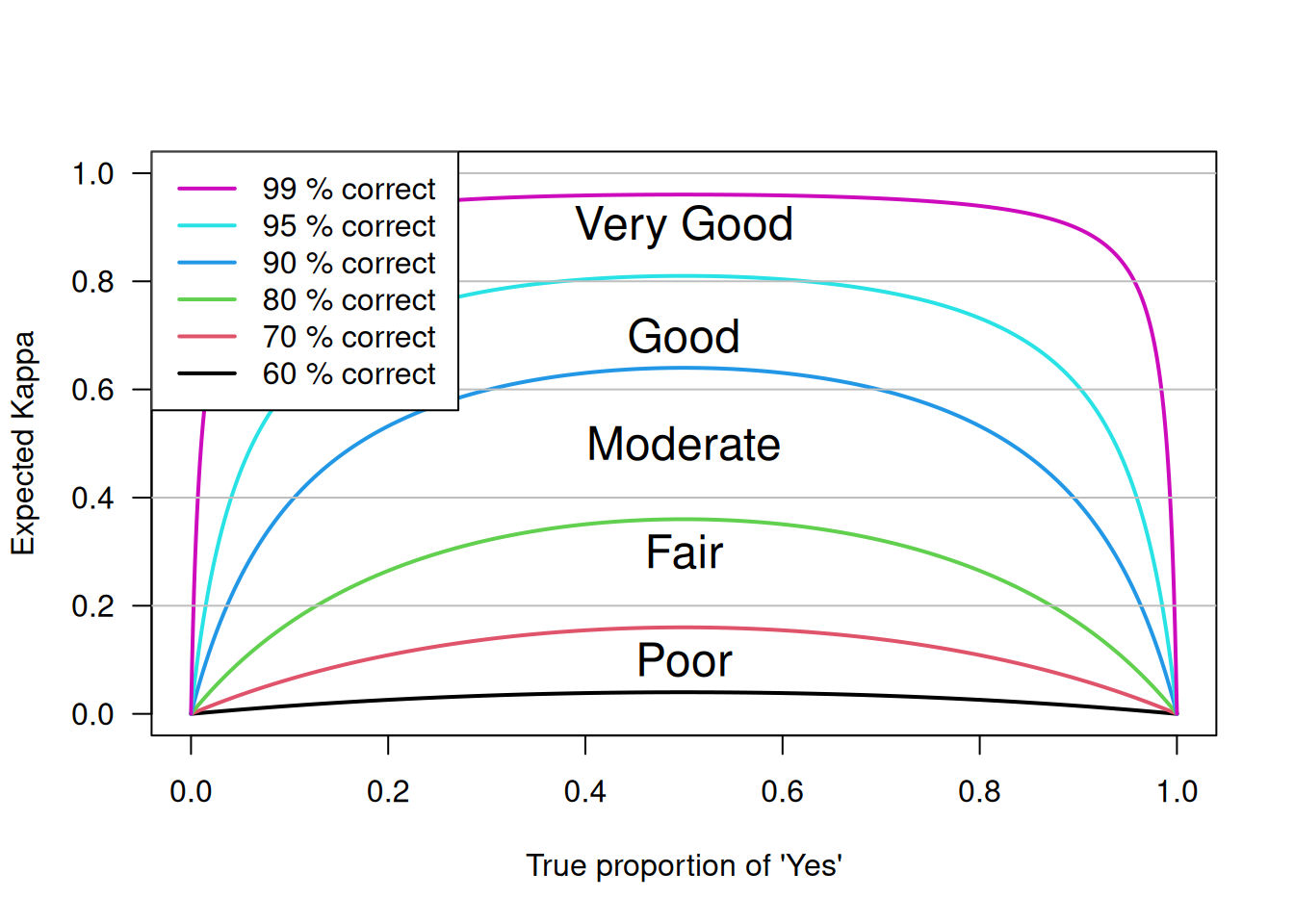
Figure 3.10: Dependence of \(\kappa\) on the true proportion of positive subjects.
| Rater 2 | |||
|---|---|---|---|
| Rater 1 | negative | positive | Total |
| negative | 84 | 4 | 88 |
| positive | 5 | 1 | 6 |
| Total | 89 | 5 | 94 |
This case shows that \(\kappa\) can be small even you have good agreement.
3.4.2 Confidence Interval for Cohen’s Kappa
The standard error of \(\kappa\) is
\[ \mbox{se}( \kappa) %% = \frac{\SE(\pobs)}{1-\pexp} = \frac{\sqrt{p_\mathrm{obs}\, (1- p_\mathrm{obs})}} {(1- p_\mathrm{exp}) \sqrt{n}}, \]
so a Wald confidence interval for \(\kappa\) can be easily computed. Using the substitution method, a Wilson interval for \(p_\mathrm{obs}\) can be used to obtain a confidence interval for \(\kappa\) with better properties. For small \(n\), bootstrap CIs might be even better, which would also incorporate uncertainty with respect to \(p_\mathrm{exp}\).
The different confidence intervals require different formats of the data: The Wald CI requires data in aggregated form (the ordinary \(2 \times 2\) table format):
## Pneumonia No pneumonia
## Pneumonia 176 240
## No pneumonia 83 1349In contrast, the bootstrap CI needs data on individual level, with each row representing the measurements from one child:
## RadiologistA RadiologistB
## 1 Pneumonia Pneumonia
## 2 Pneumonia Pneumonia
## 3 Pneumonia Pneumonia## RadiologistB
## RadiologistA No Pneumonia Pneumonia
## No Pneumonia 1349 240
## Pneumonia 83 176A Wald confidence interval is obtained conveniently using psych::cohen.kappa():
## Call: cohen.kappa1(x = x, w = w, n.obs = n.obs, alpha = alpha, levels = levels)
##
## Cohen Kappa and Weighted Kappa correlation coefficients and confidence boundaries
## lower estimate upper
## unweighted kappa 0.37 0.42 0.47
## weighted kappa 0.37 0.42 0.47
##
## Number of subjects = 1848The Wilson confidence interval requires transformation of the confidence limits of \(p_\mathrm{obs}\) to \(\kappa\):
pobsCI <- wilson(x=sum(diag(pneumonia)), n=sum(pneumonia))
pexp <- sum(diag(chisq.test(pneumonia)$expected))/sum(pneumonia)
## substitution method
round((pobsCI-pexp)/(1-pexp), 2)## lower prop upper
## 0.36 0.42 0.48A 95% bootstrap CI for Cohen’s Kappa is obtained using
biostatUZH::confIntKappa():
## Caution, used levels in weighted Kappa: No Pneumonia, Pneumonia## 2.5% 97.5%
## 0.37 0.473.4.3 Weighted Kappa
It might happen that more than two categories are of interest.
Example 3.6 The physical health of 366 subjects has been judged by a health visitor (HV) and the subject’s general practitioners (GP) (Bland, 2015). Table 3.6 shows the data (columns: HV, rows: GP).
| Poor | Fair | Good | Excellent | |
|---|---|---|---|---|
| Poor | 2 | 12 | 8 | 0 |
| Fair | 9 | 35 | 43 | 7 |
| Good | 4 | 36 | 103 | 40 |
| Excellent | 1 | 8 | 36 | 22 |
As a start, we may only consider the diagonal entries for the assessment of agreement. This is unweighted kappa, calculated with \[\begin{eqnarray*} p_\mathrm{obs}= \sum_{i=1}^{k} p_{ii}, \\ p_\mathrm{exp}= \sum_{i = 1}^k p_{i\cdot} p_{\cdot i} \end{eqnarray*}\] where \(k\) is the number of categories, \(p_{ij}\) is the observed relative frequency in cell (\(i\), \(j\)), while \(p_{i\cdot}\) and \(p_{\cdot i}\) are the corresponding row and column sums.
However, agreement for ordinal variables is better based on the weighted \(\kappa_w\) with weights of agreement \(w_{ij}\):
\[\begin{eqnarray*} p_\mathrm{obs}&=& \sum_{i=1}^{k}\sum_{j=1}^{k} w_{ij}p_{ij}, \\ p_\mathrm{exp}&=& \sum_{i = 1}^k\sum_{j=1}^{k} w_{ij}p_{i \cdot} p_{\cdot j}, \\ \kappa_w &=& \frac{p_\mathrm{obs}-p_\mathrm{exp}}{1-p_\mathrm{exp}}. \end{eqnarray*}\] The standard error of \(\kappa_w\) is also available.
Common choices for the weight are quadratic and linear weights.
- Quadratic weights:
## Poor Fair Good Excellent
## Poor 1.00 0.89 0.56 0.00
## Fair 0.89 1.00 0.89 0.56
## Good 0.56 0.89 1.00 0.89
## Excellent 0.00 0.56 0.89 1.00- Linear weights:
## Poor Fair Good Excellent
## Poor 1.00 0.67 0.33 0.00
## Fair 0.67 1.00 0.67 0.33
## Good 0.33 0.67 1.00 0.67
## Excellent 0.00 0.33 0.67 1.00Example 3.6 (continued) Analysing agreement of the health assessments from Table 3.6 with the unweighted and weighted \(\kappa\):
## Call: cohen.kappa1(x = x, w = w, n.obs = n.obs, alpha = alpha, levels = levels)
##
## Cohen Kappa and Weighted Kappa correlation coefficients and confidence boundaries
## lower estimate upper
## unweighted kappa 0.053 0.13 0.20
## weighted kappa 0.266 0.35 0.44
##
## Number of subjects = 366The function cohen.kappa()
uses quadratic weights as default. The weighted \(\kappa_w\) turns out to be
larger than the unweighted \(\kappa\).
3.5 Additional references
Literature on this topic includes Chapter 13 in Altman et al (2000) and Chapter 20 on clinical measurements in Bland (2015). Methodological discussions can be found in the Statistics Notes Altman and Bland (1996a), Altman and Bland (1996b), and Altman and Bland (1996c). Studies where the methods from this chapter are used in practice are for example Islami et al (2009), Little et al (2002), and Spector et al (1995).