Topic 7 Apoptosis and Necrosis
Apoptosis is programmed cell death that is mediated by a series of highly complex and orchestrated mechanisms. Apoptosis is characterized by cell shrinkage, membrane changes, DNA fragmentation, chromatin condensation, membrane blebbing, and the formation of apoptotic bodies.
More importantly is the fact that apoptosis does not result in inflammation and is energy dependent; it also relies on the activation of a set of cysteine processes called caspases.
Figure 7.1: Differences Between Apoptosis and Necrosis
Necrosis, on the contrary, is accidental cell death that does not require energy. During necrosis, there is cell swelling and cytoplasmic vacuole formation. Cytoplasmic contents of cells are also released into the external environment, hence leading to inflammation.
7.1 Caspases
Figure 7.2: Some Caspases Known to Science
A caspase is an aspartate-directed cysteine protease that plays a key role in the initiation and the execution of apoptosis. However, it is also important to note that not all caspases play a role in apoptosis.
Caspases are synthesized as inactive procaspases. Furthermore, caspases that are invovled in apoptosis can be divided into two classes:
Initiator caspases
These caspases are responsible for caspase activation and form the recruitment domain (i.e., CARD) or the death domain (i.e., DED).
Executioner (i.e., effector) caspases
These are not present.
7.1.1 Separation of procaspases
Figure 7.3: Separation of Pro-Caspases
Procaspases are cleaved into a large and a small subunit that together, form a heterodimer.
Two such heterodimers then form an active tetramer.
7.2 Pathways of Apoptosis
Figure 7.4: Pathways of Apoptosis
This sub-chapter explores the above graphic.
7.2.1 Extrinsic pathway
This pathway involves transmembrane receptor-mediated interactions. Such death receptors are also members of the tumor necrosis factor (i.e., TNF) receptor gene superfamily: this superfamily also shares cysteine-rich extracellular domains and have a cytoplasmic death domain (i.e., dd).
As of the time of writing, the best characterized extrinsic pathway of apoptosis are the FasL / FasR and the TNF-a / TNRF1 models.
7.2.1.1 Receptor-mediated caspase activation at the DISC
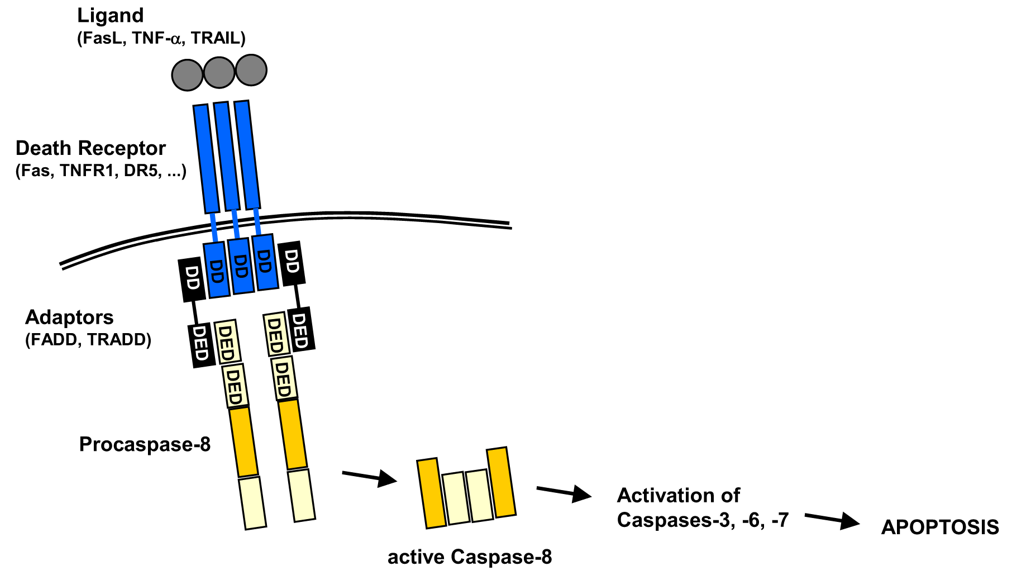
Figure 7.5: A Schematic of Caspase Activation at the DISC
Note the above graphic - also note the following abbreviations:
DD \(\rightarrow\) Death Domain
Adaptors: DD-DED (i.e., Death Effector Domain)
These include Fas-Associated protein with DD (i.e., FADD) and TRNF1-associated with DD (i.e., TRADD).
Procaspase-8 as the DED
7.2.2 Intrinsic pathway of apoptosis
This pathway involves a diverse amount of non-receptor-mediated stimuli: signals that act directly on targets within a cell that cause mitochondrial-initiated events.
During this pathway, there is also a loss of the mitochondrial transmembrane potential and an opening of the mitochondrial permeability transition pore, releasing two groups of pro-apoptotic proteins into the cytosol in the process:
Cytochrome-c and Smac/DIABLO
This group activates the caspase
AIF, endonuclease G, and CAD
This second group causes late-event DNA fragmentation.
7.2.3 Compromisation of the mitochondrial membrane
Figure 7.6: Mitochondrial Membrane Compromisation in the Intrinsic Pathway of Apoptosis
The mitochondrial membrane is compromised via the Bcl2 protein family. Because of this, we also say that the intrinsic apoptotic pathway hinges on the balance of activity between the pro and the anti apoptotic members of the Bcl-2 superfamily of proteins that regulate the permeability of the mitochondrial membrane.
Figure 7.7: Types of Apoptotic Caspases in Apoptosis
Also note the types of apoptotic caspases presented in the above graphic.
7.3 Apoptosis
Figure 7.8: Formation of the Apoptosome
The above graphic shows the formation of the apoptosome.
Figure 7.9: Sub-products of Caspase Activations
Also note the products formed from the activation of executioner caspases and substrate processing.
7.4 Apoptosis Inhibitions
Figure 7.10: Inhibiting Apoptosis
The inhibitor of apoptosis (i.e., IAP) family of proteins protects cells from killing themselves via the caspase death cascade.
IAP proteins bind to and inhibit activated caspases; some IAP proteins like Livin also encourage E3 ubiquitin ligase activity and promotes activated caspase degradation via degrading Smac/DIABLO via ubiquitination.
Smac / DIABLO promotes apoptosis via stopping IAP-caspase interactions. Hence, degrading Smac / DIABLO allows IAP family proteins to block caspase activity (hence promoting cell survival).