Chapter 4 Endocrine System II
4.1 The Thyroid Gland
The thyroid gland is the largest organ in the endocrine system; it sits right in front of the neck (and below the larynx) and secretes thyroid hormones (obviously)!
Follicular cells of the thyroid gland secrete T3 (i.e., tri-iodothyroxine) and T4 (i.e., thyroxine). Parafollicular cells secrete calcitonin
Figure 4.1: T3 and T4 Cells
Also note that the enzymes thyroglobulin and thyroperoxidase are needed to produce T3 and T4. Thyroglobulin is the precursor molecules for T3 and T4; thyroperoxidase oxidizes I- ions to form the latter two hormones:
Figure 4.2: Synthesis of Thyroid Hormones
4.1.1 Activation and transport of thyroid hormones
Out of the hormonal secretions of the thyroid, 90% of those secretions are T4; the other 10% is T3 (T4 is converted into T3 in the liver and the kidneys):
Figure 4.3: T3 and T4 Hormones
Curiously, about 0.3% of T3 hormones and 0.03% of T4 hormones are free in the bloodstream. T3 is also four times more potent than T4.
4.1.2 What do thyroid hormones do?
Thyroid hormones:
- Increase the basal metabolic rate of the organism (hence raising body heat).
- Converts glycogen into glucose.
- Increases cell sensitivity to epinephrine and norepinephrine.
Thyroid hormones are needed for the proper growth and development of organisms.
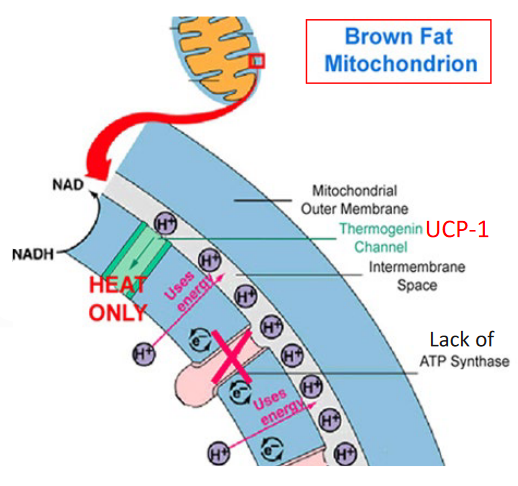
Figure 4.4: Brown Fat and Heat Production
4.1.3 More on brown fat
Brown fat contains a protein called irisin. When bound to white fat cells, these adipocytes can get converted over into beige adipocytes:
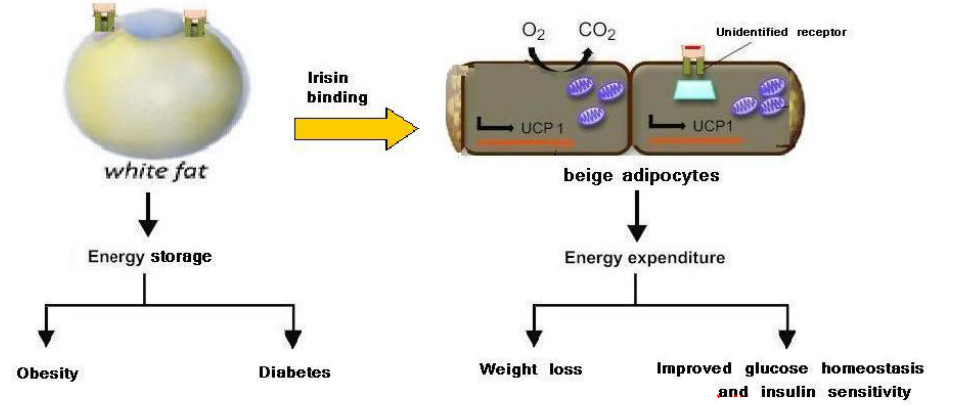
Figure 4.5: Irisin Converts White Adipocytes to Beige Adipocytes
Interestingly, irisin has also be shown to reverse Alzheimer’s disease in mice!
Irisin improves the brain’s plasticity while repairing memory defects in mice as well - it also mimics the effect of physical exercise on the brain as well!
4.1.4 Diseases from thyroid malfunctioning
Hypothyroidism (i.e., undersecretion of thyroid hormones) arises from a deficiency of iodine, an autoimmune disease, or a deficiency of TSH and / or TRH.
Goiter is a condition that develops from overstimulation of the thyroid by TSH.
Figure 4.6: Somebody with Goiter
Neonatal hypothyroidism can lead to a cretinism, where infants have severely impaired physical and mental growth.
Graves’ disease is a common result of hyperthyroidism: in this disease, the body erroneously produces thyroid-stimulating immunoglobulins (i.e., TSIs) that act against TSH receptors. TSIs cannot be regulated via negative feedback:
Figure 4.7: Somebody with Graves’ Disease
4.2 Calcium in the Body
The distribution of Calcium is as such in the body:
Figure 4.8: Ca2+ Distribution in the Body
Calcium concentrations in the body are vital:
Hypocalcemia leads to increased neuromuscular activity; it may lead to muscle cramps and in severe cases, spasms of the respiratory muscles (leading to death by suffocation).
Hypercalcemia reduces excitability and cardiac arrhythmias.
4.3 Parathyroid glands
These are four embedded glands in the side of the thyroid glands that secrete parathyroid hormone (i.e., 84 amino acid polypeptide).
Figure 4.9: Parathyroid Glands
PTH is also the most important hormone in the control of calcium concentrations in the blood; its secretion is stimulated by a decreased amount of blood calcium.
4.3.1 Effects of PTH on various biological structures
4.3.1.1 In bones…
PTH promotes the transfer of calcium ions from bone fluid to blood plasma. The dissolution of calcium phosphate crystals into phosphate and calcium ions (slow) also happens here.
4.3.1.2 In kidneys…
PTH encourages calcium ion reabsorption and phosphate elimination.
4.3.1.3 In the intestines…
Calcium ion and phosphate ion absorption are encouraged; vitamin D is also activated in the kidneys.
4.3.1.4 Consequences of PTH malfunction
4.3.1.4.1 PTH hyposecretion
This is very rarely a result of genetic factors or autoimmune disorders. More often than not, this is often a result of a thyroidectomy gone wrong; the complete absence of PTH is fatal (because hypocalcemia causes increased neuromuscular activity).
4.3.1.4.2 PTH hypersecretion
This causes kidney failure or malignancy.
4.3.2 Vitamin D
Vitamin D is also known as calcitriol and is either found in the diet or made in the liver or the skin.
In the liver, vitamin D is converted into 25-hydroxy vitamin D; in the kidneys, vitamin D is converted into 1,25-dihydroxy vitamin D. Vitamin D also promotes the absorption of calcium ions and phosphate ions in the small intestine (as mentioned previously), and also increases bone responsiveness to PTH.
A lack of vitamin D is responsible for causing osteomalacia (i.e., softening of the bones) and rickets (i.e., childhood osteomalacia).
4.3.3 Calcitonin
This is a hormone that is secreted by the parafollicular cells of the thyroid. Its secretion is controlled by high blood calcium concentrations.
Calcitonin is an antagonist to PTH: it inhibits osteoclast activity, delays calcium absorption in the intestines, and also increases the amount of calcium excreted from the body. Calcitonin has been used to treat osteoporosis.
Funnily enough, estrogen stops bone resorption and also promotes bone deposition. Hence, a low amount of estrogen in the system can lead to increased bone fractures and weaker bones - this happens more in post-menstrual women due to altered estrogen levels.
Osteoporosis in often known as the “silent thief” because of its lack of symptoms.
4.4 Adrenal Glands
Figure 4.10: Adrenal Glands
These glands are embedded in a capsule of fat just above the kidneys. The adrenal glands are made up of the adrenal cortex and the adrenal medulla.
4.4.1 Adrenal cortex
Figure 4.11: Zones of the Adrenal Medulla
Each zone in the cortex has a unique set of enzymes for synthesizing steroid hormones.
4.4.2 Aldosterone
This hormone stimulates the kidneys to reabsorb sodium and secrete potassium. Secondary effects of this hormone include the retention of water and the expansion of extracellular fluid volume.
Aldosterone is essential for life!
4.4.2.1 Regulating aldosterone secretion
A decrease in sodium concentrations in the body and blood pressure activates the Renin-Angiotensin-Aldosterone System (i.e., RAAS).
Similarly, a raise in the body’s potassium levels act directly on the adrenal cortex to produce aldosterone:
Figure 4.12: Activation of the RAAS
4.4.2.2 When aldosterone secretions don’t go right…
4.4.2.2.1 Aldosterone hypersecretions
This is usually a result of a tumor (i.e., Conn’s syndrome); however, a secondary cause of this could be decreased blood flow to the kidneys (hence resulting in aldosterone hypersecretion).
4.4.2.2.2 Aldosterone hyposecretion
Addison’s disease is an autoimmune disease - all layers of the adrenal cortex are affected!
Furthermore, coginental adrenal hyperplasia may also result as a consequence of a genetic defect in 21-hydroxylase or 11\(\beta\)-hydroxylase.
4.4.3 Glucocorticoids
Cortisol is the main glucocorticoid for most mammals (including humans) - corticosterone serves the same function in rodents, birds, amphibians, and reptiles; the short form of this hormone is cortisone.
Nevertheless, corticosterones are oftne used for making drugs that mimic the effect of cortisol (e.g., dexamethasone).
4.4.3.1 Functions of glucocorticoids
These hormones play major roles in stress adaptations. They also play immunosuppressive effects (i.e., they suppressthe inflammatory response).
They also have several metabolic effects:
- Stimulation of gluconeogenesis
- Inhibting glucose uptake
- Reducing protein stores and encouraging proteolysis
- Lipolysis in the limbs and promoting fatty acid oxidation
- Lipogenesis in the face and in the torso.
4.4.3.2 Cortisol hypersecretion
Cushing’s syndrome may be a consequence (know the following symptoms);
Figure 4.13: Cushing’s Syndrome
4.4.3.3 Cortisol hyposecretion
Addison’s disease and congenital adrenal syndrome may from as a result. Hyperpigmentation can also form.
NB: Pro-opiomelanocortin (i.e., POMC) is the precursor molecule for making ACTH.
4.5 Adrenal Androgens
DHEA (dehydroepiandrosterone) is the sole biologically significant androgen from the adrenal cortex, but it is still much weaker than testosterone.
DHEA may play significant roles in females; it is responsible for:
- Growth of pubic hair
- Female sex drive
- Growth spurt
Its stimulation is brought on by ACTH and is negatively regulated by GnRH instead of CRH.
4.5.1 Hypersecretions
This can lead to androgenital syndrome and is commonly caused by a deficiency in 21-hydroxylase.
This is also associated with the hyposecretion of aldosterone and cortisol.
4.6 Adrenal Medulla
The medulla produces 80% epinephrine and 20% norepinephrine (collectively known as catecholamines).
Norepinephrine is a neurotransmitter in post-ganglionic neurons; Epinephrine and norepinephrine receptors are adrenergic, of which the \(\alpha\) and \(\beta\) are the major classes:
- \(\alpha 1\)-receptors activate the phospholipase C pathway.
- \(\alpha 2\)-receptors inhibit the adenylate cyclase pathway.
- \(\beta 1\) and \(\beta 2\) receptors bind to G proteins.
4.6.1 What does epinephrine do?
This hormone is responsible for the fight or flight response in humans: it increases the rate and the strength of heart contractions (via \(\beta 1\) receptors), it dilates blood vessels in the heart and in the muscles via the \(\beta 2\) vessels, and it constricts all other blood vessels via the \(\alpha 1\) receptor.
Epinephrine also promotes gluconeogenesis and glycogenesis (in the liver and in the skeletal muscles) and lipolysis. Glucagon secretions are encouraged and insulin secretions are stopped.
Note that alpha cells secrete glucagon; beta cells secrete insulin.
4.7 What Does Insulin Do?
Insulin receptors act on skeletal muscles, liver, and adipose tissue.
Insulin promotes the entry of glucose, amino acids, and fatty acids into the cell to encourage anabolic processes. Consequently, proteolysis, proteolysis, and glycogenolysis is discouraged, hence decreasing levels of glucose, fatty acids, and amino acids in the blood.
4.7.1 Regulation of Insulin Secretion
High plasma levels of glucose and amino acids are responsible for insulin secretions. Glucogan and gastric-inhibitory-peptide (i.e., GIP) are also responsible for encouraging the secretion of insulin.
Somatostatin and sympathetic activation of the nervous system are responsible for downregulating insulin secretions.
4.7.2 Type I and II diabetes mellitus
Figure 4.14: Traits of Type I and II Diabetes Mellitus
Furthermore, free fatty acids in the bloodstream may also inhibit GLUT4 translocation, hence causing insulin resistance.
As a general rule of thumb, there is an immune destruction of insulin receptors or a decreased expression of insulin receptors that all contribute to decreased insulin sensitivity.
Figure 4.15: Insulin Signalling
4.8 Endocrine Control in Fuel Metabolism
There are several key tissues in fuel metabolism:
- Liver (glycogenesis, glycogenolysis, and glyconeogenesis)
- Adipose tissue (stores fat)
- Muscle (stores amino acids)
- Brain (uses glucose as its only energy source, but cannot store glycogen).
In response to stress, epinephrine and cortisol are released, hence raising blood glucose and fatty acid levels.
Growth hormone also increases blood glucose, fatty acid, and amino acid uptake (alongside protein synthesis).