3 Seminar Two
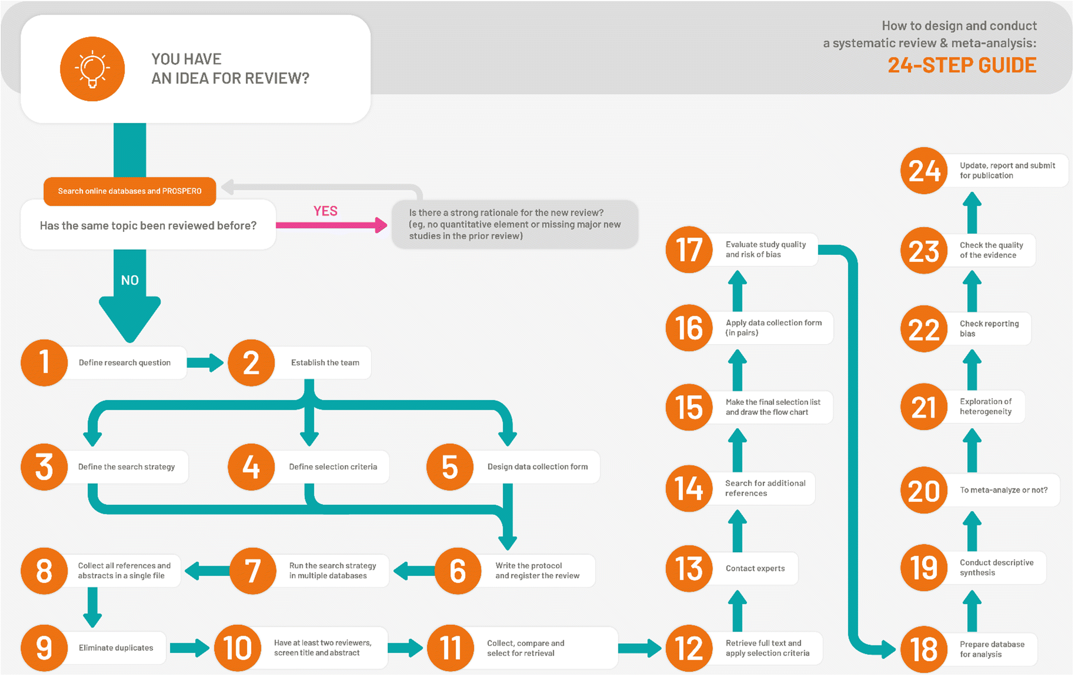
How to design and conduct a systematic review - 24 step process
In the lecture this week you will have learnt about advanced search strategies. This is a really important step in undertaking a systematic review, because without capturing all, or at least most, of the relevant studies on a research question it is highlight unlikely you will be able to answer it. As such, this step takes careful planning and evaluating before starting the review. In your theory lesson earlier this week you will have been introduced to the 12 step process of developing a search. In your seminar today you will be using this approach to develop a search strategy for your review (assessment).
3.1 Task 1
You will/should have formulated your systematic review question last week using the PICOS or SPIDER now develop a hypothetical experimental trial which would best answer your question:
3.1.1 Task 1A
Simple overview:
Open a new word document, copy the table below and provide a brief description of each element of your research question (PICOS)
| Item | Description |
|---|---|
| Study design | — |
| Population | — |
| Intervention | — |
| Comparison | — |
| Outcome | — |
3.1.2 Taske 1B
Detailed overview:
Now using the headings below, along with the examples to help, draft a more detailed overview of your hypothetical trial
Title: The Effectiveness of Resistance Exercise Compared to Waitlist Control on Balance and Strength in Older Adults: A Randomized Controlled Trial
Objective: To investigate the impact of resistance exercise compared to a waitlist control group on balance and strength in older adults.
Hypothesis: We hypothesize that older adults who participate in resistance exercise will demonstrate significant improvements in both balance and strength compared to those in the waitlist control group.
Study Design: This study will employ a randomized controlled trial design with two groups: the resistance exercise group and the waitlist control group.
Participants: Inclusion criteria:
- Age: 65 years or older.
- Generally healthy, without any major medical conditions that would prevent participation in an exercise program.
- Sedentary lifestyle or minimal physical activity.
- No recent (< 6 months) history of resistance exercise training.
Exclusion criteria:
- Significant cognitive impairment that would affect the ability to follow instructions.
- Severe musculoskeletal limitations or injuries that would prevent participation in resistance exercise.
Procedure:
Recruitment: Older adults meeting the inclusion criteria will be recruited through community centers, senior organizations, and local advertisements.
Randomization: Participants will be randomly assigned to either the resistance exercise group or the waitlist control group, using a computer-generated randomization sequence.
Resistance exercise group:
- Participants in this group will undergo a supervised resistance exercise program.
- The program will consist of two to three sessions per week for a duration of 12 weeks.
- Each session will include a warm-up period, resistance exercise targeting major muscle groups, and cool-down exercises.
- Exercise intensity and progression will be individually tailored based on participants’ abilities and goals.
- Qualified exercise professionals will supervise and monitor the exercise sessions.
Waitlist control group:
- Participants in this group will maintain their regular daily activities and will not receive any structured exercise intervention during the 12-week study period.
- They will be informed that they will have an opportunity to participate in the resistance exercise program after the study is completed.
Outcome measures:
- Balance: Balance will be assessed using standardized tests such as the Berg Balance Scale or the Timed Up and Go test.
- Strength: Muscle strength will be measured using dynamometers or one-repetition maximum (1RM) tests for specific muscle groups.
Assessments:
- Baseline assessments will be conducted before randomization.
- Follow-up assessments will be conducted immediately after the 12-week intervention period for both groups.
Data analysis:
- Statistical analyses, such as independent t-tests or non-parametric equivalents, will be used to compare the outcomes between the resistance exercise group and the waitlist control group.
- The significance level will be set at p < 0.05.
Ethical Considerations:
- Informed consent will be obtained from all participants.
- The study protocol will be approved by the relevant ethical review board.
- Participants will be assured of confidentiality and the right to withdraw from the study at any time without consequences.
Conclusion: The findings from this hypothetical trial will provide insights into the effectiveness of resistance exercise compared to a waitlist control group in improving balance and strength among older adults. These results can inform the development of evidence-based exercise interventions for promoting healthy aging and functional independence in the older population.
3.2 Task 2
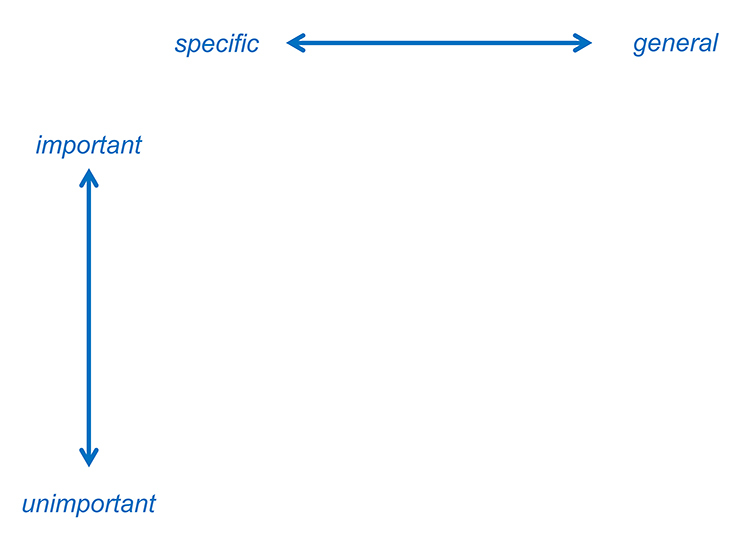
Schema to determine the optimal order of search
In your word document copy the table below and search each of your key terms, ranking each in terms of importance and recording the number of retrievals that are returned from each database. Then using the image above determine where they fall on the important/unimportant axis and on the specific/general axis.
Below are the quick links to the databases
N.B., if you are struggling to recall how to navigate these pages (re)-review materials from reviewing scientific literature (specifically week 3) from last year
| Item | Search term | Importance(rank) | CINAHL(n) | MEDLINE(n) | SportDiscus(n) | Total (n) |
|---|---|---|---|---|---|---|
| Study design | — | — | — | — | — | — |
| Population | — | — | — | — | — | — |
| Intervention | — | — | — | — | — | — |
| Comparison | — | — | — | — | — | — |
| Outcome | — | — | — | — | — | — |
3.3 Task 3A
Download the [search log template] to your own Onedrive and populate it with the terms that are most important and specific to those that were unimportant and general. For each of these (top level/concrete) terms enter the number of retrievals from each database and begin to list alternative terms.
N.B., if you are struggling to recall what concrete and alternative terms are (re)-review materials from reviewing scientific literature (specifically week 3) from last year
3.4 Task 3B
Use the CINAHL subject headings feature to identify and add any alternative terms you might have missed to your search log. You can also add the MeSH heading if it is appropriate, however these are beyond the scope of this module and have not been taught.
N.B., MeSH headings are beyond the scope of this module but you can find out lots about how they are used by databases online. This tutorial from EBSCO connect is a good place to start.
3.5 Task 4
Once you have completed your log and exhausted all of the alternative terms, you can now play around with different combinations of these terms, to see the impact of adding or subjecting a particular term has. When adding terms has little change on your retrievals you can be more confident that you have captured all the literature. Once you reach ‘optimisation’ you have your final search string. Record this in your protocol and save your search log.
How to change the sensitivity or specificity of a search
NB: You can find an example of an advanced search strategy log in appendix one (p. 12) of Heine M, Rietberg MB, Amaral Gomes E, Evenhuis E, Beckerman H, van de Port I, de Groot V, Kwakkel G, van Wegen EEH.Exercise therapy for fatigue in multiple sclerosis (Protocol).Cochrane Database of Systematic Reviews 2022, Issue 12. Art. No.: CD015274.DOI: 10.1002/14651858.CD015274.
3.6 Task 5
Complete the ‘searcher’ section of the PRESS (Peer review of Electronic Search Strategies) and send this and your search log to someone in your group for peer review. N.B., it is expected that you will act as a peer reviewer for one of your classmates searches in return.
3.7 Task 6
Update the search strategy (databases and interfaces, and Query strings) section and add the completed PRESS checklist as an appendix to the protocol document you saved last week to your own OneDrive.
3.8 Additional reading
Aromataris, E., & Riitano, D. (2014). Constructing a search strategy and searching for evidence. Am J Nurs, 114(5), 49-56.