7 Seminar Six
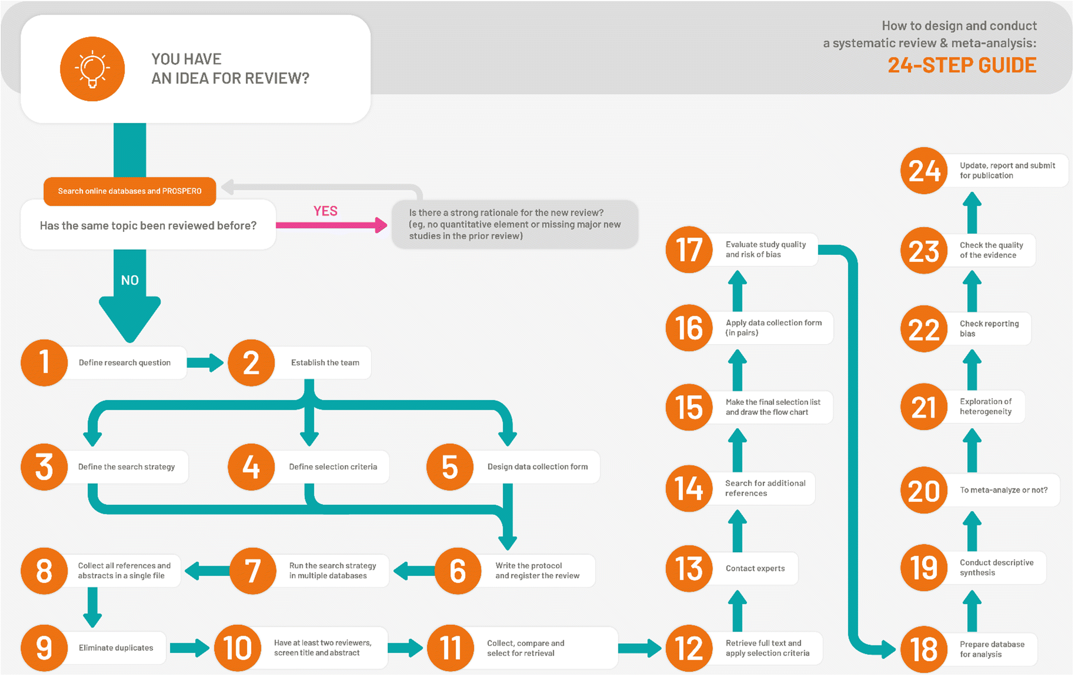
How to design and conduct a systematic review - 24 step process
In the lecture this week you will have learnt about bias in randomised controlled trialss, and quality appraisal techniques to evaluate the risk of bias in individual studies. In this seminar you will learn to apply these quality appraisal tools and make judgements on the certainty of individual study findings.
7.1 Task 1
Open the Risk of Bias (RoB 2.0) and save it to your own OneDrive. Open the ‘RoB 2 Assessment Form’ using the ‘Intro’ tab.
Use the quality appraisal tool to assess the risk of bias in the article below: Schedler, S., Brueckner, D., Hagen, M., & Muehlbauer, T. (2020). Effects of a traditional versus an alternative strengthening exercise program on shoulder pain, function and physical performance in individuals with subacromial shoulder pain: a randomized controlled trial. Sports, 8(4), 48.
NB: An alternative way of conducting the RoB 2.0 is to use the word template for completing the assessment
7.2 Task 2
using the ‘Results’ and ‘Summary’ tab, compare and discuss your quality appraisal with one of your peers, where you have disagreements/inconsistencies in your responses to signalling questions discuss your reasons for awarding the decision and together reach a consensus.
7.3 Task 3
How does this quality assessment influence your confidence in the study findings reported? (i.e., increase/decrease your confidence/certainty in the study findings)
7.4 Task 4
Add the following statement to your systematic review protocol, if you are including randomised studies: ‘Randomised controlled trials of human participants will be assessed using the RoB 2.0 tool (Sterne et al., 2019) for bias arising from randomisation, in deviations from intended interventions, in missing outcome data, in measurement of the outcome and in the selection of the reported results. Each item will be judged either at ’high risk of bias’, ‘low risk of bias’ or ‘some concerns of risk of bias’. Studies in which all domains are judged ‘low risk of bias’ are considered ‘low risk of bias’ overall, studies that raise ‘some concerns of risk of bias’ in at least one domain, but have no ‘high risk of bias domains’ are classified as ‘some concerns of bias’ overall, and studies that judge at least one domain to be of ‘high risk of bias’, or have multiple domains of ‘some concerns of risk of bias’ that lowers confidence in the result are deemed to be ‘high risk of bias’ overall.’
7.5 Additional reading
https://training.cochrane.org/rob-2-learning-live-webinar-series