6 Seminar Five
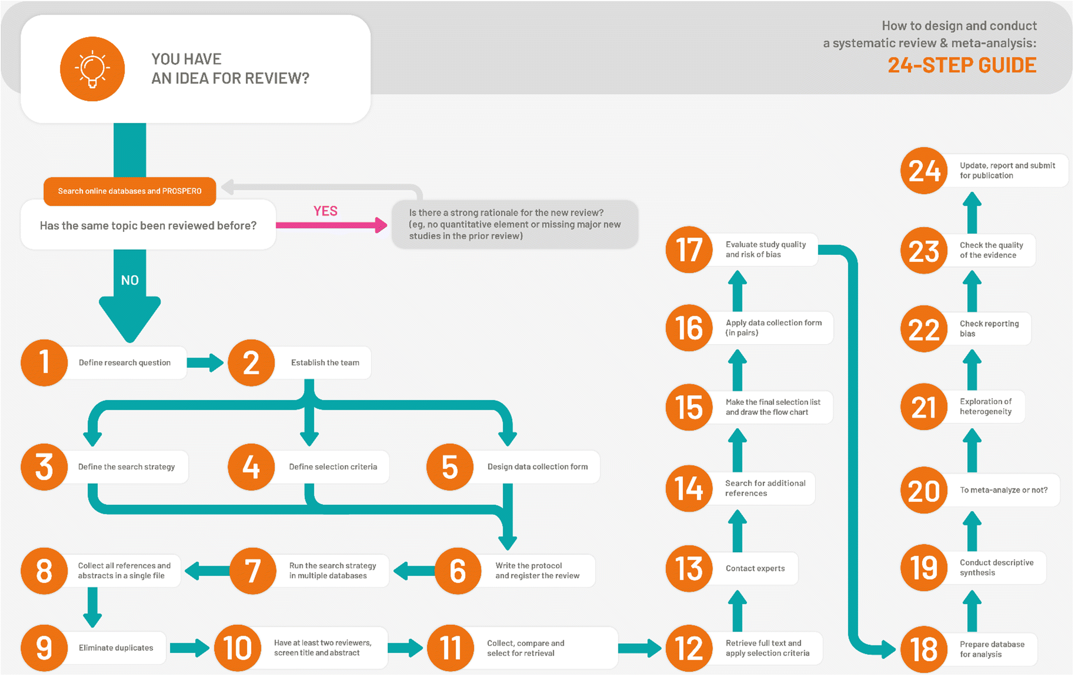
How to design and conduct a systematic review - 24 step process
In the lecture this week you will have learnt about bias in non-randomised and observational study designs, and quality appraisal techniques to evaluate the risk of bias in individual studies. In this seminar you will learn to apply these quality appraisal tools and make judgements on the certainty of individual study findings.
6.1 Task 1
Download the Downs and Blacks quality appraisal tool and save it to your OneDrive.
Use the quality appraisal tool to assess the risk of bias in the article below:
Hewage, K., Fosker, S., Leckie, T., Venn, R., Gonçalves, A. C., Koulouglioti, C., & Hodgson, L. E. (2023). The Hospital to Home study (H2H): smartwatch technology-enabled rehabilitation following hip fracture in older adults, a feasibility non-randomised trial Future Healthc J, 10(1), 14-20.
6.2 Task 2
Compare and discuss your quality appraisal with one of your peers, where you have disagreements/inconsistencies in your responses to signalling questions discuss your reasons for awarding the decision and together reach a consensus.
6.3 Task 3
How does this quality assessment influence your confidence in the study findings reported? (i.e., increase/decrease your confidence/certainty in the study findings)
6.4 Task 4
Add the following statement to your systematic review protocol if you are including non-randomised studies: ‘The evaluation of the studies’ methodological quality included in the review will be conducted according to the quality index for randomised and observational studies proposed by Downs and Black, which has a checklist of 26 items. The index has five subscales (reports; external validity; internal validity—bias; internal validity—confused and power) whose items are scored from 0 or 1, except for one item in the report subscale, scored from 0 to 2 and the single power item scored from 0 to 5. The maximum total score of the methodological quality assessment index is 32 points’