11 Seminar Ten
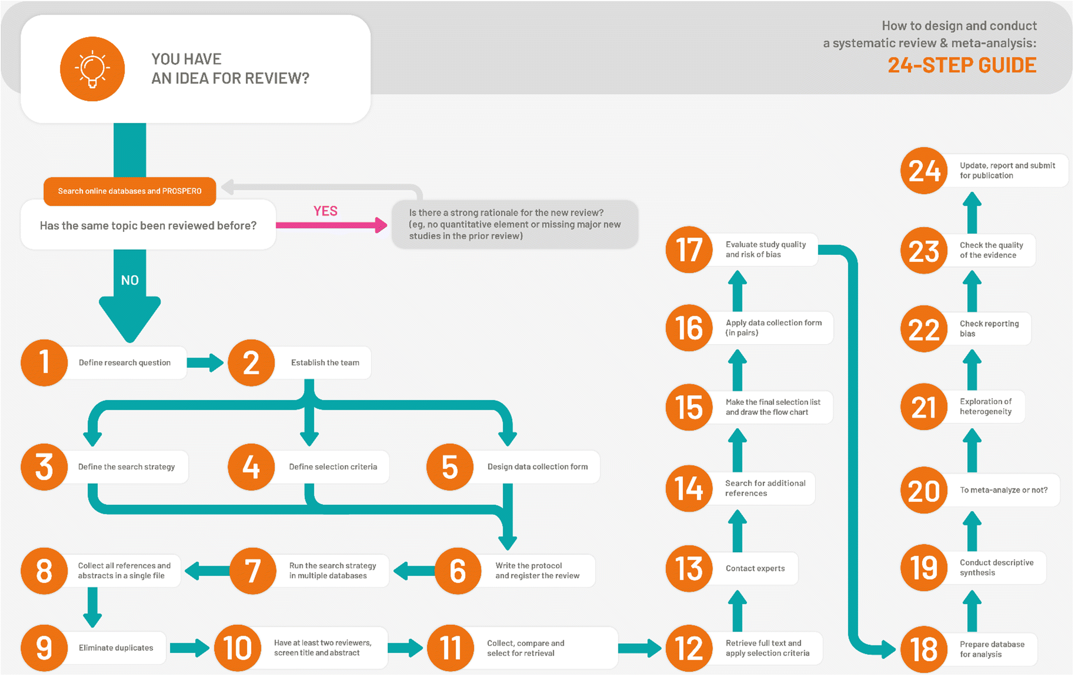
How to design and conduct a systematic review - 24 step process
In the lecture this week you will have learnt about the reporting guidelines of the methodological section of your manuscript (assessment). In today’s session you should continue to conduct your systematic review and should use these guidelines to write up your methods section.
Use your protocol to perform the following tasks (remember however that the protocol should have been written in future tense whereas the methodology for your manuscript/assessment should be written in past tense)
11.1 Task 1
Write up your study eligibility using the example below:
Example: ‘We included randomized controlled trials (RCTs) comparing exercise interventions in at least one treatment arm with control conditions, ensuring the control group receives the same additional treatments when exercise is combined with others. Randomized crossover trials were included but treated as parallel-group trials, with data analyzed up to the crossover point. We excluded abstract-only publications, non-randomized single-arm studies, observational studies, cluster RCTs, and historically controlled studies. Studies were eligible if they recruited adults (aged 18+) with non-specific lower back pain of any type and severity, regardless of gender, ethnicity, or geographical location. Interventions encompass structured, multi-joint exercise interventions conducted at least once weekly for two or more consecutive weeks, both supervised and unsupervised alongside standard care, while co-interventions are considered if the effects of exercise could be isolated. Exclusions included targeted physiotherapy interventions, self-administered exercise, and studies measuring exercise as a dependent variable rather than as an independent one.’
11.2 Task 2
Write up your search strategy using the example below:
Example: ’Our search strategy to identify pertinent studies for inclusion in our analysis focused on published, peer-reviewed journal articles. We conducted systematic searches of two primary databases: MEDLINE via PubMed (covering the years 1946 to 2023) and CINAHL via EBSCO (spanning from 1981 to 2023). To comprehensively capture relevant studies, we crafted a search string comprised of four distinct categories of controlled vocabularies and free-text terms. These categories encompassed exercise, osteoarthritis, muscle mass, and balance. We did not impose any language or publication date restrictions, ensuring inclusivity of studies across various languages and publication years (see Supplementary File X for more details). Our initial database searches were initiated in September 2022, and subsequent updates were conducted in December 2023 to incorporate the latest research findings.
Study eligibility was judged against the pre-defined inclusion and exclusion criteria. Two authors (ES, RW) independently screened the titles and abstracts of articles for eligibility. In cases of disagreement the two authors discussed each case and tried to reach a consensus. If a consensus could not be reached a third reviewer was consulted (CP). If inadequate information was available in the title and abstract to determine eligibility it was included for next stage (full text) review. The same two authors (ES, RW) independently reviewed the full text of articles that were included based on the title and abstract screening. In cases where there was disagreement about the eligibility of an article, the two authors aimed to reach a consensus. If a consensus could not be reached a third reviewer (CP) was consulted. The authors were not blind to study authors, journals, or results. A screening log was maintained and the number of excluded studies and their reason for exclusion recorded.
11.3 Task 3
Write up your data collection procedures using the example below:
Example: Data extraction for included studies was performed two authors (ES and RW) using a pre-piloted system, whereby one author extracted data into a table, and another validated the data extraction. Extracted data included study details (e.g., authors, year), study design, population (e.g., sample size, demographic information, health status), intervention details (e.g., duration, exercise frequency, intensity, time, type), comparator conditions, outcome details (e.g., definition, assessment method), and statistical measures (e.g., post-intervention means and standard deviation).
11.4 Task 4
Write up your quality appraisal procedures using the example below:
Example: The quality of non-randomised studies included in this review were assessed according to the quality index for randomised and observational studies proposed by Downs and Black, which has a checklist of 26 items. The index has five subscales (reports; external validity; internal validity—bias; internal validity—confused and power) whose items are scored from 0 or 1, except for one item in the report subscale, scored from 0 to 2 and the single power item scored from 0 to 5. The maximum total score of the methodological quality assessment index is 32 points. The quality of randomised studies included in this review were assessed using the RoB 2.0 tool for bias arising from randomisation, in deviations from intended interventions, in missing outcome data, in measurement of the outcome and in the selection of the reported results. Each item was judged either at ‘high risk of bias’, ‘low risk of bias’ or ‘some concerns of risk of bias’. In studies where all domains were judged as ‘low risk of bias,’ they were considered ‘low risk of bias’ overall. Studies that raised ‘some concerns of risk of bias’ in at least one domain, but had no ‘high-risk of bias domains,’ were classified as ‘some concerns of bias’ overall. Finally, studies that judged at least one domain to be of ‘high risk of bias’ or had multiple domains with ‘some concerns of risk of bias’ that lowered confidence in the results were deemed to be ‘high risk of bias’ overall.