The following graphic shows the proportion of energy expenditure during the average human’s exercise:
Energy Content of Fat
There are several advantages in storing energy in the form of fatty acids:
Carbon in fatty acids is almost completely reduced - this is in contrast to carbons in sugars or amino acids! Because of this, oxidizing fatty acids will yield more energy in the form of ATP than any other form of carbon (e.g., sugars, amino acids, nucleic acids, etc).
Furthermore, fatty acids are not as hydrated as monosaccharides: this enables them to pack more closely during storage!
Fatty Acid Pathways
Fatty acid release from adipose tissues
Hormones trigger the release of fatty acids from adipose tissues. Lipolysis (the movement of stored fat) is homonally controlled by cyclic AMP (cAMP).
This release is also mediated by epinephrine during stressful situations and periods of fasting.
Though, it is important to note that other hormones are responsible for regulating this release under other conditions.
Mobilization of fats from food
Alkaline pancreatic secretions secreted into the duodenum (i.e., the first portion of the small intestine) raises the pH of the mixture and activates triacylglycerol hydrolysis by pancreatic lipase and esterases.
However, the aforementioned reactions depend on bile salts. Bile salts serve to emulsify triglycerols and enhance the activity of pancreatic lipase and esterases.
A chylomicron is a structure formed from newly formed triacylglycerols (from fatty acids and glycerol) aggregating in lipoproteins.
Bile salts are derivatives of cholesterol and are synthesized in hepatocytes (i.e., liver cells). Cholesterol is first converted into cholic acid and chenodeoxycholoic acid - these acids then get conjugated to glycine or taurine to yield anions called bile salts!
And, just in case one is curious, the general structure for a plasma protein is shown below: a hydrophobic inner core of cholsterol esters (in yellow) and a surface of hydrophilic phosphate heads.
Furthermore, lipoproteins can also be classified according to the following table:
Chylomicrons also have several fates (as shown in the below graphic):
Chylomicrons (and VLDL) are also broken down in the capilaries for hydrolysis of triacylglyceride to glycerol and free fatty acids (to be consumed by cells).
Lipase is a member of the serine esterase family (like trypsin) with the three amino acids at the active site: serine, histidine, and aspartate.
Fatty Acid Oxidation
The moment fatty acids enter a cell, three processes must occur:
First, the fatty acid must be activated by conversion to fatty acyl-CoA:
Thereafter, the fatty acyl-CoA (which is unable to cross the plasma membrane by simple diffusion) must be transported from the cytosol into the mitochondrial matrix.
Carnitine - an amino-oxy acid - undergoes an ester-forming reaction with fatty acyl-CoA, hence yielding an acyl-carinitine ester that moves across the membrane into the mitochondria via facilitated diffusion.
Finally, the \(\beta\)-oxidation pathway was elaborated and built on by three scientists:
Franz Knoop found that fatty acids are first degraded by oxidation at the \(\beta\)-carbon, followed by the cleavage of the \(C_\alpha - C_\beta\) bond.
Albert Lehninger found that the above pathway occurred in the mitochondria.
F. Lynen and E. Reichart found that the two-carbon unit released is acetyl-CoA.
In mammalian cells, \(\beta\)-oxidation occurs in mitochondria; however, a similar pathway also happens in the peroxisome!
\(\beta\)-oxidation in the mitochondria provides energy to the organism, but peroxisomal \(\beta\)-oxidation in the peroxisome is responsible for shortening long-chain fatty acids (these are poor substrates for mitochondrial \(\beta\)-oxidation).
Fatty acid activation by acyl-CoA synthetase
In the case of long-chain fatty acids, this reaction takes place at the outer membranes in higher eukaryotes before the fatty acids enter the mitochondria.
The overall \(\Delta G^\omicron\) is about -0.8 kJ / mol, so this reaction is highly favorable and easily reversible.
However, the pyrophosphate produced in this reaction is rapidly hydrolyzed by inorganic pyrophosphatase to two molecules of phosphate. The net \(\Delta G^\omicron\) is about -33.6 kJ / mol.
Hence, we say that pyrophosphate concentrations are maintained at a low concentration in the cell. The aforementioned conversion is highly promoted!
Carnitine cycle for transporting fatty acyl-CoAs into mitochondria
Long-chain fatty acyl-CoA derivatives must first be converted into acylcarnitine derivatives.
Carnitine acyltransferase I is associated with the outer mitochondrial membranes and catalyzes the formation of O-acylcarnitine. The latter product is then transported across the inner membrane via a translocase.
The acetylcarnitine is then passed to carnitine acyltransferase II to to the matrix-side of the inner membrane. The fatty acyl group is then transferred to CoA (to form acyl-CoA): this generates free carnitine which can return to the membrane via the translocase.
Beta-oxidation of fatty acyl-CoA
Note that the total energy output from fatty acid catabolism is measured in the amount of ATP produced.
Each round of \(\beta\)-oxidation produces one molecule of NADH, one molecule of FADH2, and one molecule of acetyl-CoA.
Oxidation of the acetyl-CoA via the citric acid cycle then generates an additional two molecules of NADH and one molecule of FADH2.
Also note…
Fatty acids are generally classified according to the following guidelines:
- very long chain fatty acids: more than 20 carbon atoms
- long-chain fatty acids: 12 to 20 carbon atoms
- medium-chain fatty acids: 6 to 12 carbon atoms
- short-chain fatty acids: 4 carbon atoms
The below table shows the amount of energy released from the oxidation of different fatty acids:
Oxidizing Fatty Acids with Odd-Numbered Chains
\(\beta\)-oxidation of odd-carbon fatty acids yields a three-carbon propionyl-CoA molecule.
This pathway involves an initial carboxylation at the \(\alpha\)-carbon of propionyl-CoA to produce a D-methylmalonyl-CoA. This reaction is also catalyzed by propionyl-CoA carboxylase.
This pathway also invovles an ATP-driven carboxylation of biotin, followed by a nucleophilic attack by the \(\alpha\)-carbanion of propionyl-CoA in a stereospecific manner.
Oxidizing Unsaturated Fatty Acids
Unsaturated fatty acids also undergo \(\beta\)-oxidation, but there are two additional mitochondrial enzymes involved:
- an isomerase
- a novel reductase
The above two enzymes are responsible for handling cis double bonds.
Observe the \(\beta\)-oxidation of linoleic acid:
As observed above, the \(\beta\)-oxidation takes place in three steps:
Enoyl-CoA isomerase converts the cis-\(\Delta^3\) double bond to a trans-\(\Delta^2\) double bond to permit one more round of \(\beta\)-oxidation.
This results in a cis-\(\Delta^4\) enoyl-CoA, which is then subsequently oxidized to trans-\(\Delta^2\) and cis-\(\Delta^4\) by acyl-CoA dehydrogenase.
The enzyme 2,4-dienoyl-CoA reductase yields the trans-\(\Delta^3\) product which is then converted into the trans-\(\Delta^2\) form by enoyl-CoA isomerase.
A Side-by-Side Comparison of Fatty Acid Beta Oxidation and Fatty Acid Biosynthesis
Note that the carboxylation of acetyl-CoA to malonyl-CoA is irreversible and is the first committed step of fatty acid biosynthesis.
The aforementioned reaction is also catalyzed by acetyl-CoA carboxylase (ACC): an enzyme with a biotin prosthetic group and is regulated by phosphorylation, allosteric modification, and induction / repression of its genes.
Citrate activates ACC by causing individual enzyme molecules to polymerize.
The end product palmitoyl-CoA inhibits ACC.
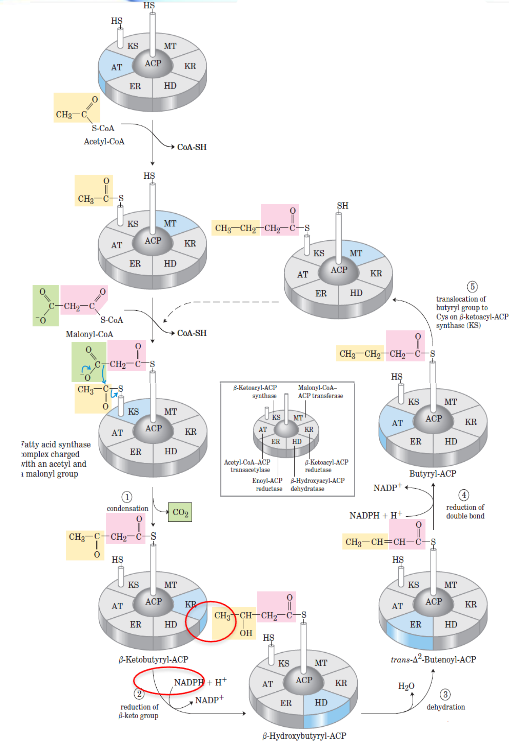
Fatty acid synthase complex
In E. coli and some plants, the seven-active sites (six enzymes and an ACP) for fatty acid synthesis reside in seven separate polypeptides.
In this complex, each enzyme has its active site near the preceding and succeeding enzymes of the sequence.
The flexible pantethine arm of the ACP can reach all active sites; it also carries a growing fatty acyl chain from one site to the next. Intermediates are not released from the enzyme complex until the finished prouct has formed.
Reaction cycle for biosynthesis of fatty acids
The \(\beta\)-ketoacyl-ACP synthase catalyzes decarboxylative condensation of the acyl group with malonyl-ACP to produce an acetoacetyl-ACP.
There are two key differences between fatty acid biosynthesis and fatty acid oxidation:
- Alcohol formed in biosynthesis is of the D-configuration, not the L-configuration.
- The reducing coenzyme here is NADPH; NAD+ and FAD are oxidants in the catabolic pathway.
Fatty Acid Elongation
Shorter chain fatty acids are easily made if it is released before reaching 16 carbons (in length).
Longer chain fatty acids are made via special elongation reactions that occur on the surface of the endoplasmic reticulum (ER) and in the mitochondria. Elongation in the ER involves successive condensations of malonyl-CoA with acetyl-CoA and NADPH-associated reductions.
Mitochondrial reactions involve the addition of acetyl units.
These reactions are pretty much a reversal of fatty acid oxidation reactions. However, the exception is that NADPH is utilized in the saturation of the double bond instead of FADH2.