Chapter 11 Amino Acid Metabolism
The primary purpose of amino acids are to:
- Act as a monomer in protein synthesis
- Act as a substrate for biosynthetic reactions of different compounds (e.g., NO, hormones, nicotinamide, heme, purines, and pyrimidines)
Our diet is the source of our amino acid intake!
11.1 An Overview of Amino Acid Metabolism
Free amino acids throughout the body occupies a central position in protein and amino acid metabolism:
Figure 11.1: Amino Acid Pool
Amino acids released from digestion are first passed from the gut through the hepatic portal vein to the liver; most amino acids are used for the synthesis of proteins in the liver and in other tissues!
Note that excess amino acids may be converted into glucose or triacylglycerol:
Figure 11.2: Amino Acid Metabolism in the Liver
During periods of fasting, amino acids are released from muscle tissue; glutamine is thereby oxidized by various tissues (including lymphocytes, stomach tissues, and kidney tissues). Alanine and other amino acids travel to the liver; their carbons are then converted into glucose and ketone bodies. The nitrogen is converted into urea to be excreted by the kidneys.
Figure 11.3: Amino Acid Metabolism During Periods of Fasting
The carbon skeleton of amino acids (after the amino acid degraded) can be broken down into CO2 and H2O or converted into glucose, acetyl-CoA, or ketone bodies.
11.2 Amino Acid Catabolism (nitrogen atom)
11.2.1 Transanimation
Most aminotransferases use glutamate / \(\alpha\)-ketoglutarate as one of two \(\alpha\)-amino / \(\alpha\)-keto acid pairs involved. Transanimation is a route for redistributing nitrogen from amino acids andd requires a PLP cofactor (from vitamin B6). Also, no free ammonium is released during any part of transanimation!
However, note that lysine, proline, and threonine cannot undergo transanimation reactions!
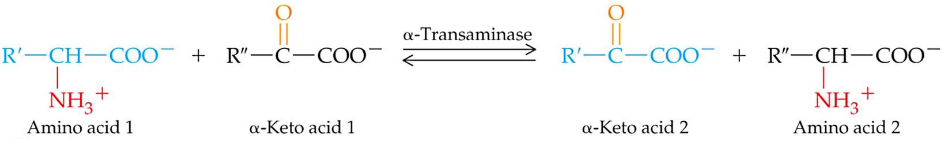
Figure 11.4: Transanimation Reaction
Serum glutamate-oxaloacetate transaminase (SGOT) catalyzes the interconversion between oxaloacetate and aspartate.
Figure 11.5: SGOT and SGPT
Serum glutamate-pyruvate transaminase (SGPT) catalyzes the interconversion of pyruvate and alanine.
11.2.2 Vitamin B6
As mention in chapter 2, PLP is the prosthetic group for many enzymes that catalyze a variety of reactions involving amino acids: transanimation, racemization, decarboxylation, and side-chain eliminatino or replacement just to name a few.
11.2.3 How does transanimation work?
In the first step of almost all PLP-dependent reactions, the aldehyde group of PLP forms an imine with a lysine side chain:
Figure 11.6: First Stepe of Almost All PLP-Dependent Reactions
Generally, the next step is the exchange of the imine; the nitrogen on an amino acid replaces lysine’s nitrogen during the imine linkage. This substrate-coenzyme complex is stabilized by a hydrogen bond that forms between PLP and the imine nitrogen.
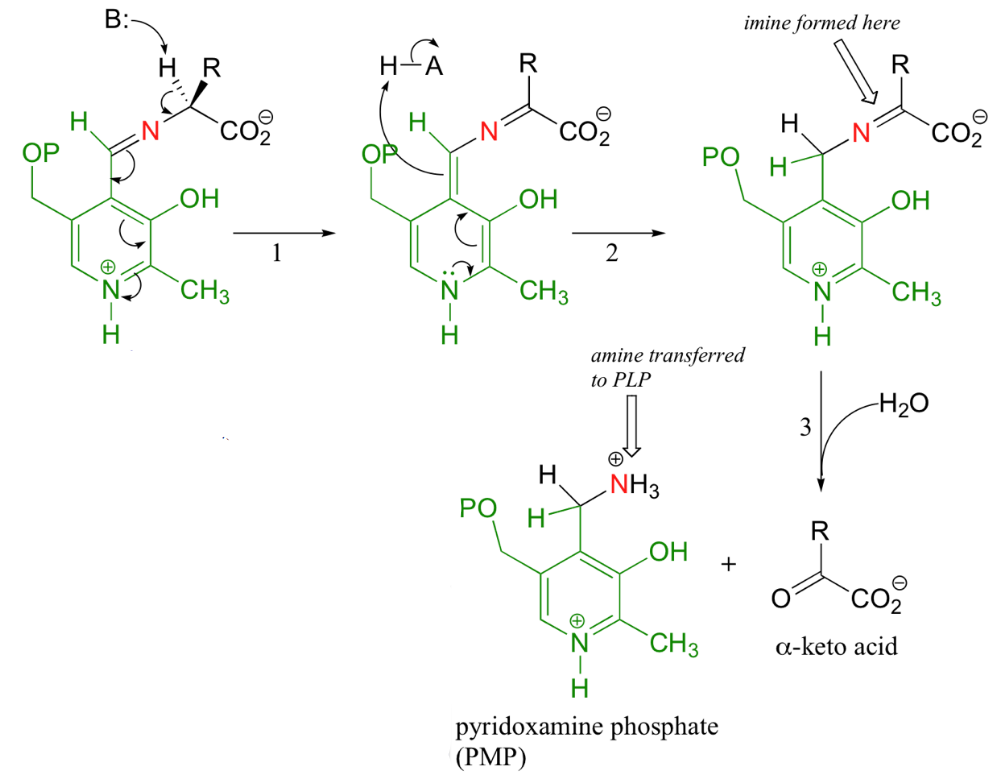
Figure 11.7: Steps of Transanimation
Thereafter (1), the alpha proton from the PLP-amino acid complex is abstracted. In transanimation reactions, this step is then followed by a reprotonation of the aldehyde carbon of the PLP (2), hence resulting in a new carbon-nitrogen double bond between the \(\alpha\)-carbon and the nitrogen of the original amino acid.
The imine is then hydrolyzed (3) - the nitrogen is then removed from the amino acid to form an \(\alpha\)-keto acid that can be further degraded. The coenzyme that is now present is a pyridoxamine phosphate (PMP).
11.2.3.1 Fates of a nitrogen atom
11.2.3.2 Deanimation
The liberation of an amino group from an amino acid may result in the production of urea from ammonia. This step is either oxidative or non-oxidative.
11.2.3.2.1 Non-oxidative deanimation
The amino acids serine, threonine, cysteine, and histidine undergo this type of deanimation to form their corresponding \(\alpha\)-keto acid.
The enzyme dehydratase catalyzes the deanimation of serine and threonone (PLP dependent); desulfurases deanimate cyteine (also PLP dependent), and histidases catalyze the deanimation of histidine.
11.2.3.2.2 Oxidative deanimation
The glutamate amino group is removed as an ammonium ion to yield \(\alpha\)-ketoglutarate.
This type of deanimation is catalyzed by glutamate dehydrogenase and use NAD+ and NADP+ as a coenzyme.
Figure 11.8: Oxidative Deanimation
Such a reaction typically occurs in the kidneys and livers.
During amino acid degradation, aminotransferase works in conjunction with glutamate dehydrogenase. The combined action of a transanimase and glutamate dehydrogenase is referred to as transdeanimase:
Figure 11.9: Transdeanimase in Action
11.2.4 Alternatives to deanimation
Amino acids can be oxidized by FMN and FAD; these oxidation reactions are catalyzed by L-amino acid oxidase and D-amino oxidase respectively. Both enzymes are present in the liver and the kidney:
Figure 11.10: Alternatives to Deanimation
11.3 The Krebs-Henseleit Urea Cycle
Figure 11.11: Krebs-Henseleit Urea Cycle
11.3.1 Step 1
The enzyme Carbamoyl phosphate synthase (CPS-I) catalyzes the reaction convers ammonia from gluatamate into carbamoyl phosphate. The latter product then combines with ornithine to form citruline (a reaction that is catalyzed by ornithine transcarbamoylase).
Figure 11.12: Step 1 of the Krebs-Henseleit Urea Cycle
11.3.2 Step 2
This reaction is driven by the dephosphorylation of ATP to AMP and pyrophosphate - this is followed by the additional exergonic hydrolysis of pyrophosphate.
The enzyme argininosuccinate synthetase is responsible for catalyzing this reaction:
Figure 11.13: Step 2 of the Krebs-Henseleit Cycle
11.3.3 Step 3
The argininosuccinate is then cleaved to form arginine and fumarate in an elimination reaction that is catalyzed by argininosuccinate lyase.
Arginine is the immediate precursor of urea:
Figure 11.14: Step 3 of the Krebs-Henseleit Cycle
11.3.4 Step 4
The guanidinium group is then cleaved to form ornithine and urea in a reaction that is catalyzed by arginase:
Figure 11.15: Step 4 of the Krebs-Henseleit Cycle
11.3.5 Regulation of the Krebs-Henseleit urea cycle
The cycle itself is regulated by carmaboyl phosphate synthetase (i.e., CPS-I).
All mammals have two versions of this enzyme:
- CPS-I (the mitochondrial version)
- CPS-II (the cytosolic version)
CPS is subject to allosteric activation by N-acetylglutamate and produced from the combination of acetyl-CoA and glutamate (alongisde increased concentrations of arginine).
11.3.6 Disorders of the urea cycle:
Observe the following table:
Figure 11.16: Disorders of the Urea Cycle
11.4 Amino Acid Catabolism (carbon atom)
Amino acids that form acetyl CoA or acetoacetyl CoA and contribute to the formation of fatty acids or ketone bodies are called ketogenic.
Amino acids that are degraded into pyruvate or Krebs’ cycle intermediates are called glucogenic.
Some amino acids can also be glucogenic and ketogenic as different parts of their carbon chains form different products.
11.5 Biosynthesis of Amino Acids
Pyruvate, oxalatoacetate, glutamate, and \(\alpha\)-ketoglutarate.
11.5.1 Nonessential amino acids
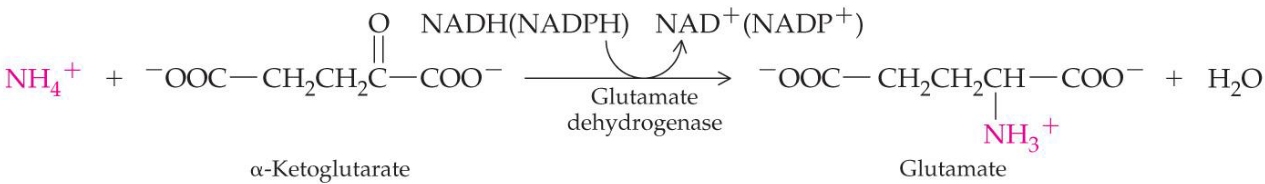
Figure 11.17: Glutamate Synthesis
Gluatamate can be synthesized from NH4+ and \(\alpha\)-ketoglutarate via reductive animation - this, however, requires NADH or NADPH.
This reaction is carried out by glutamate dehydrogenase. Note that glutamate is a major donor of amino groups in reactions; \(\alpha\)-ketoglutarate is a major acceptor of amino groups.
11.5.1.1 Making serine
Figure 11.18: Making Serine
First, the OH group is oxidized to a keto-group, yielding 3-phosphohydroxypyruvate.
3-phosphohydroxypyruvate then undergoes a transanimation reaction with glutamate to form 3-phosphoserine and \(\alpha\)-ketoglutarate.
3-phosphoserine is then hydrolyzed to give serine and Pi.
11.5.1.2 Making glycine
Serine can be turned into glycine via serine hydroxymethyltransferase:
Figure 11.19: Making Glycine
This reaction requires two cofactors:
- PLP
- Tetrahydrofolate
11.5.1.3 Making cysteine
Bacteria and plants can synthesize cysteine by incorporating H2S and by using serine as a carbon source. Some bacteria can condense H2S with serine directly via a pyridoxal phosphate-dependent enzyme.
Plants and most microorganisms use O-acetylserine as the substrate to react with H2S:
Figure 11.20: Cysteine Synthesis in Plants and Most Microorganisms
11.5.1.4 Making tyrosine
Tyrosine is non-essential as it can be synthesized from phenylalanine (an essential amino acid):
Figure 11.21: Tyrosine Synthesis
11.5.2 Vitamin B9 (i.e., folic acid)
Figure 11.22: Structure of Folate
Folate has three main components:
- pterin (black)
- p-aminobenzoic acid moiety (pink)
- glutamate (blue)