Chapter 10 Glycoprotein, Isoprenoid, and Steroid Metabolism
10.1 Glycerophospholipid Metabolism
Phospholipids are lipids that encompass both glycerophospholipids and sphingomyelins - both of which are constituents of the cell’s membrane.
Phospholipids too are precursor molecules for eicosanoids and signalling molecules.
10.1.1 Pathways in glycerophospholipid biosynthesis
Figure 10.1: Pathways of glycerophospholipid biosynthesis
The major pathways are shown in green. Pathways found in bacterial and eukaryotic cells are shown in purple. Other reactions are confined to Eukaryotic cells.
NB: Note that:
- DHAP = Dihydroxyacetonephosphate
- DAG = diacylglycerol
- AdoMet = S-adenosylmethionine
Glycerolipid synthesis in eukaryotes begins with the formation of phosphatidic acid - a molecule that may be formed from glycerol, diacylglycerol, or dihydroxyacetone phosphate.
The enzyme glycerokinase catalyzes the phosphorylation of glycerol to form glyceraldehyde-3-phosphate, which is then acylated at both the first and second positions to yield phosphatic acid.
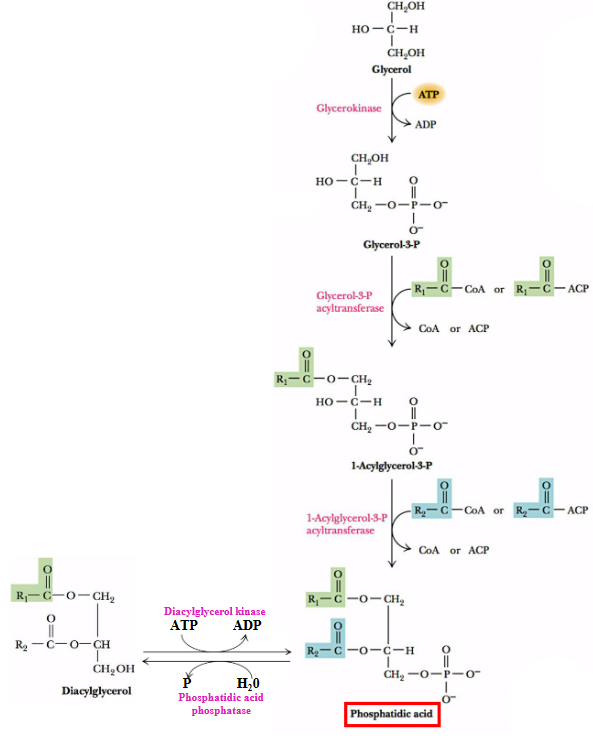
Figure 10.2: Precursors for Making Phosphatidic Acid
Also note that dihydroxyacetone phosphate can be reduced into glycerol-3-phosphate by glycerol-3-phosphate dehydrogenase.
10.1.2 Principal precursors of glycerolipids in eukaryotes
In eukaryotic organisms, phosphatidic acid is converted into diacylglycerol or to cytidine diphosphodiacylglycerol (i.e., CDP-diacylglycerol).
Diacylglycerol is a precursor molecule for synthesizing triacylglycerol, phosphatidylethanolamine, and phosphatidylcholine.
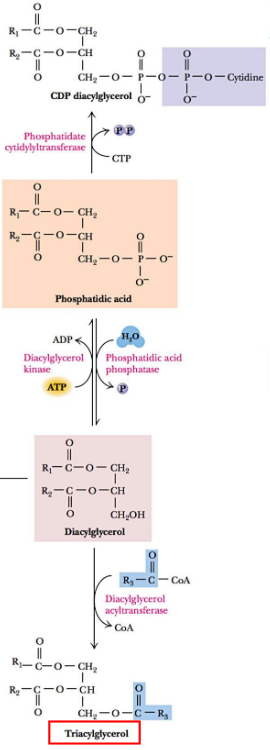
Figure 10.3: Precursors for Synthesizing Glycerolipids
Triacylglycerol is primarily made in adipose tissue, the liver, and the intestines and serves to store energy. Triacylglycerol biosynthesis the in liver and adipose tissues is catalyzed by diacylglycerol acyltransferase: an enzyme bound to the cytoplasmic portion of the endoplasmic reticulum.
10.1.2.1 Triacylglycerol biosynthesis in the intestines
Triacylglycerols from one’s diet are broken down into 2-monoacylglycerols by certain lipases.
Figure 10.4: Dietary Cholesterol Digestion
Acyltransferases then acylate the molecule 2-monoacylglycerol to produce new triacylglycerols.
10.1.3 Phosphatidylethanolamine and phosphatidylserine interconversions
All mammals synthesize phosphatidylserine (i.e., PS) in a Ca2+ ion-dependent reaction that involves an aminoalcohol exchange.
Figure 10.5: PS and PE Interconversion
Phosphatdiylserine decarboxylase is associated with the endoplasmic reticulum and will accept phosphatidylethanolamine (PE) and other phospholipid substrates - this enzyme also converts PS to PE and vice versa.
10.1.4 CDP-diacylglycerol as a precursor of glycerolipids in eukaryotes
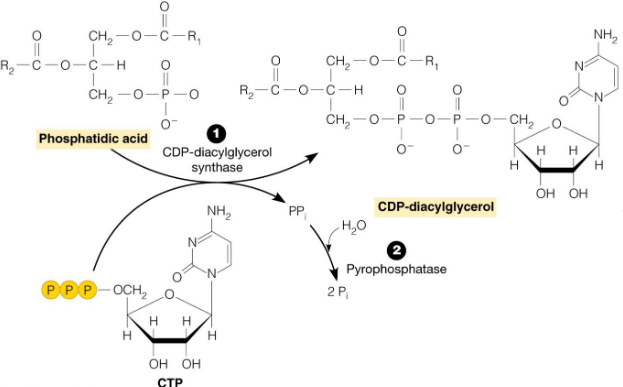
Figure 10.6: CDP-diacylglycerol Catalyzing CTP and Phosphatidic Acid Reaction
The reaction of CTP and phosphatidic acid is catalyzed by CDP-diacylglycerol in reaction 1 (shown above). The hydrolysis of pyrophosphate (by pyrophosphotase) drives the reaction to the right (i.e., to the products side).
CDP-diacylglycerol (derived from phosphatidic acid) is also used by eukaryotes as a precursor for other important phospholipids, including but not limited to cardiolipin, phosphatidylinositol, and phosphatidylglycerol:
Figure 10.7: CDP-diacylglycerol as a Precursor Molecule for Other Phospholipids
10.2 Sphingolipid Synthesis
10.2.1 Ceramide
The condensation of palmitoyl-CoA and serine is reduced by NADPH, yielding sphingamine in the process:
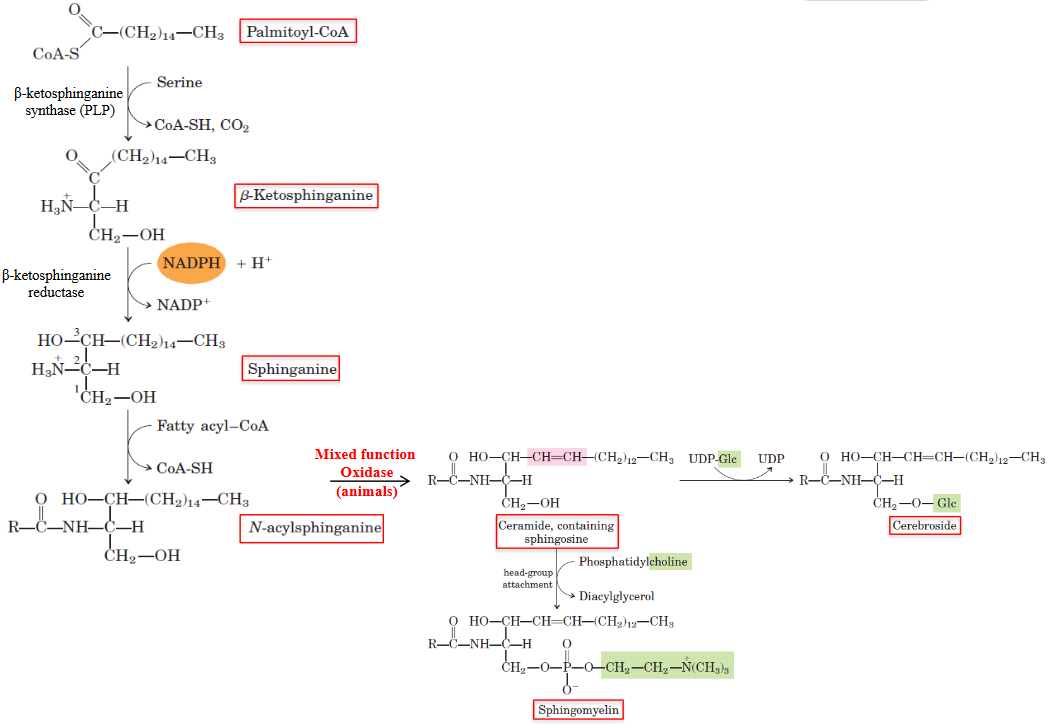
Figure 10.8: Ceramide Synthesis
Sphingamine is then acylated to N-acylsphingamine (a ceramide).
Ceramide is the “building block” for all other sphingolipids. As an example, sphingomyelin is produced when phosphocholine is transferred from phosphatidylcholine.
Glycosylating ceramides yields cerebrosides (i.e., galactosylceramide - these make up 15% of all myelin sheath structures).
10.3 Ganglioside Synthesis
Cerobrosides that contain one or more sialic acid moieties are called gangliosides.
The general form of ganglioside synthesis is illustrated below for the ganglioside GM2:
Figure 10.9: Ganglioside Synthesis
10.4 Eicosanoids and Arachidonic Acid
Eicosanoids are derived from 20-carbon fatty acids; in humans, arachidonic acid is the precursor molecule of eicosanoids:
Figure 10.10: Arachidonic Acid
10.4.1 Arachidonic acid
Arachidonic acid is the precursor molecule for prostaglandins (PG), thromboxanes (Tx), and leukotrienes.
Thromboxanes were first discovered in thrombocytes; leukotriene C was first discovered in polymorphonuclear leukocytes (a class of white blood cells) and was named after the white blood cells and its triene structure (three conjugated double bonds).
Figure 10.11: Product Syntheses from Arachidonic Acid
PGH synthase is a key step in the synthesis of prostaglandins. Aspirin inhibits the COX-1 and COX-2 forms of PGH synthase, hence aspirin’s anti-inflammatory activities.
10.4.2 Cyclooxygenation of arachidonic acid
All prostaglandins are cyclopentanoic acids that have been derived from arachidonic acid.
Biosynthesis of prostaglandins are driven by prostaglandin endoperoxide II synthase (PGHS or cyclooxygenase - COX). The enzyme that converts arachidonic acid into PGH2 has two distinct activities: a COX and a glucathione-dependent hydroxyperoxidase (POX).
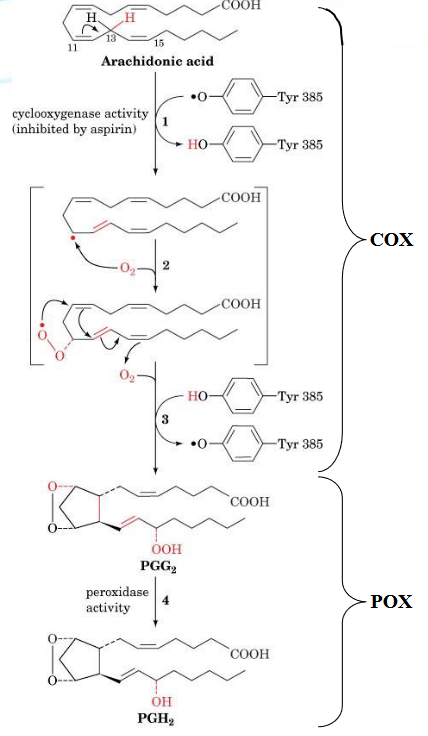
Figure 10.12: Arachidonic Acid Conversion into PGH2
Furthermore, PGH2 can also be converted into a myraid of other biologically-significant molecules:
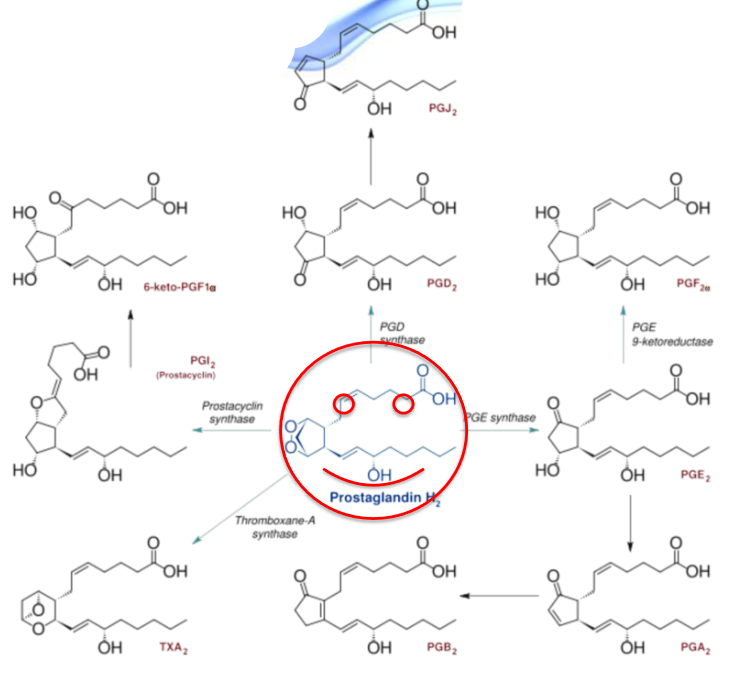
Figure 10.13: PGH2 Products
10.4.3 Aspirin’s effects on prostaglandin synthesis
Aspirin exerts its anti-inflammatory effects by inhibiting prostaglandin synthesis via acting on the PGH synthase (aspirin O-acetylates Ser350 on the enzyme).
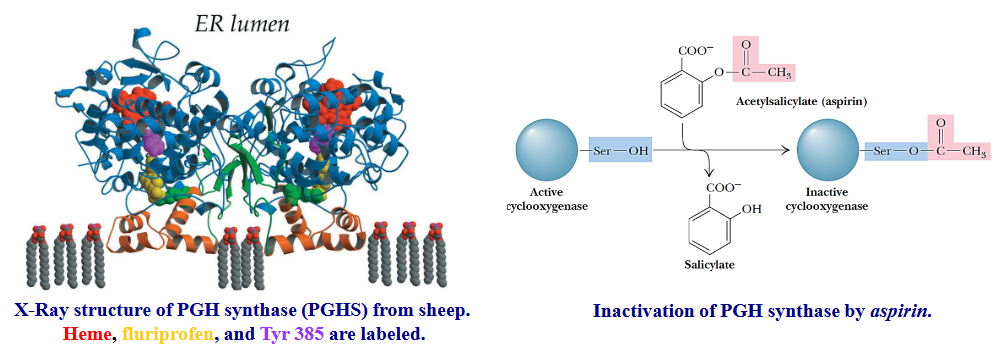
Figure 10.14: Aspirin Action
10.5 Cholesterol Synthesis and Metabolism
Steroids are a class of lipids that are classified as isoprenoids because their structures are closely related to that of isoprenes’:
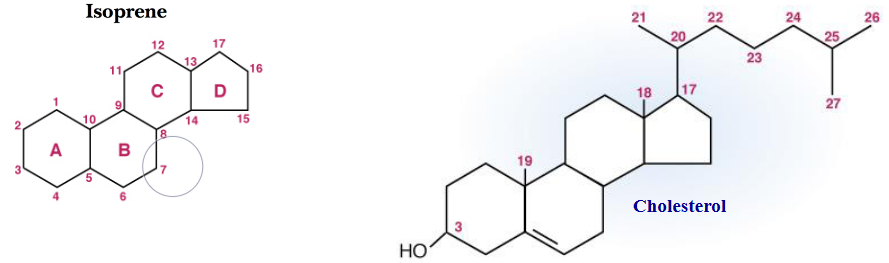
Figure 10.15: Isoprene and Cholesterol Structure
As seen above, all steroids contain four fused rings: A, B, C, and D.
Cholesterol plays an important role in mammalian biochemistry - not only is it a component of certain membranes, but it is also a precursor of steroid hormones and and bile salts.
That said, all of the carbons in cholesterol are derived from acetate and can be broken down into three steps:
- Conversion of C2 fragments to a C6 isoprenoid precursor.
- Converting six C6 mevalonates via activated C5 intermediates into a C30 squalene.
- Cyclization of squalene and its transformation to C27 cholesterol.
Figure 10.16: All Carbons in Cholesterol are from Acetate
10.5.1 Synthesizing mevalonate
This happens in the cytosol:
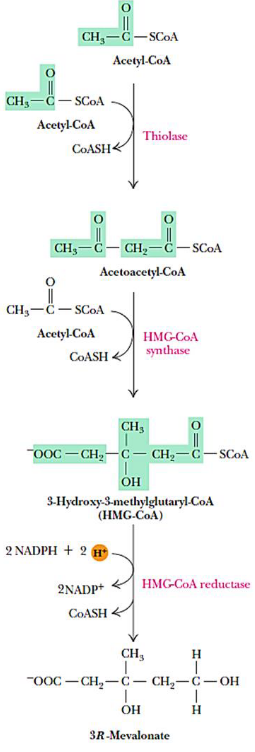
Figure 10.17: Mevalonate Synthesis
The rate-limiting step is catalyzed by HMG-CoA reductase; HMG-CoA undergoes two NADPH-dependent reductions to produce 3R-mevalonate.
10.5.2 Synthesizing isopentyl pyrophosphate
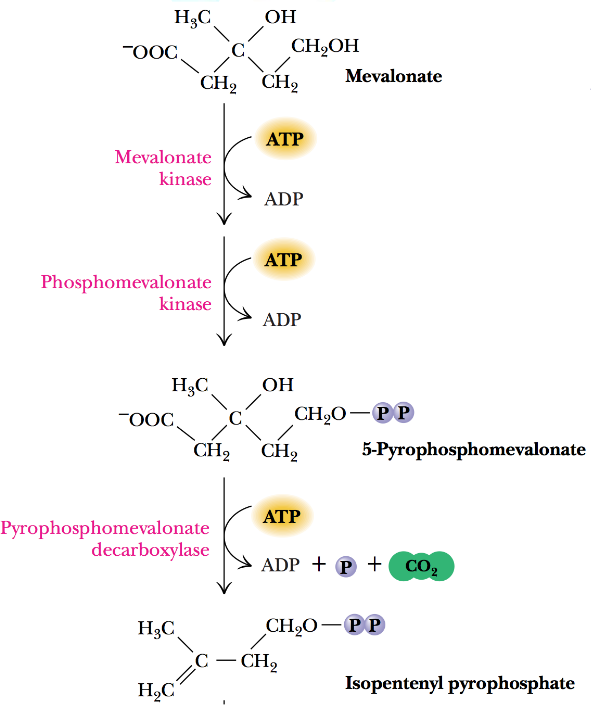
Figure 10.18: Isopentyl Pyrophosphate Synthesis
While not shown in the above graphic, mevalonate is converted into two key 5-carbon intermediates:
- Isopentyl pyrophate
- Dimethylallyl pyrophosphate
These two products then form farnesyl pyrophosphate before being yielded to form squalene.
10.5.3 Squalene formation
Figure 10.19: Squalene Formation
In the first step, the isomerization of the double bond present in isopentyl pyrophosphate yields dimethylallyl pyrophosphate. The condensation of the latter two five-carbon products then yield geranyl pyrophosphate; addition of another five-carbon isopentyl group yields farnesyl pyrophosphate.
All steps in the above graphic release pyrophosphate - this drives the reaction forward.
10.5.4 Synthesizing cholesterol from squalene
Squalene monooxygenase - an enzyme bound to the endoplasmic reticulum - converts squalene to squalene-2,3-epoxide. This reaction needs FAD and NADPH (as coenzymes) and requires O2.
2,3-oxidosqualene lanosterol cyclase catalyzes the next reaction: a series of 1,2 shifts of hydride ions and methyl groups.
10.5.5 Cholesterol synthesis from squalene
The enzymes responsible for this are all in the ER:
Figure 10.20: Cholesterol Synthesis from Squalene
10.6 Fates of Cholesterol
A small fraction of cholesterol is made in the liver, but incorporated into the membranes of hepatocytes; however, much of it is exported in one of these three forms:
- Blood cholesterol
- Bile acids
- Cholesteryl esters
Cholesterol is also used as a precursor for synthesizing steroid hormones and as a precursor for vitamin D.
10.6.1 Making bile salts
An \(\alpha\)-hydroxyl group is formed at the 7th carbon of cholesterol. This is also the rate-limiting step in bile salt synthesis.
Figure 10.21: Bile Salt Synthesis
10.6.2 HDL fates
Figure 10.22: HDL Fates
Nascent HDL is made in the liver and intestinal cells. It exchanges proteins with chylomicrons and VLDL.
First, HDL picks up cholesterol from cell membranes and converts it into cholesterol esters by lethicin-cholesterol acyltransferase (LCAT).
HDL also transfers CE to VLDL in exchange for triacylglycerol - the cholesterol ester transfer protein (CETP) mediates this exchange.
10.6.3 Cholsterol as a Precursor of Steroid Hormones
Synthesis of steroid hormones first begin with the desmolase reaction - this converts cholesterol to pregnenolone. Desmolase is found in the mitochondria of tissues that synthesize steroids.
Thereafter, pregnenolone is transported from the mitochondria to the endoplasmic reticulum where OH oxidation and migration of the double bond yields progesterone.
Figure 10.23: Steroid Hormone Synthesis
Pregnenolone synthesis happens in the adrenal cortex and is activated by the adrenocorticotropic hormone (i.e., ACTH) - a 39 amino acid long residue that is secreted by the anterior pituitary gland. Progesterone is also the precursor molecule for synthesizing other sex hormone steroids and corticosteroids.
10.6.4 Regulating cholesterol synthesis
The rate-limiting step in the conversion of HMG-CoA to mevalonate. Additionally, cholsterol synthesis is regulated by:
- PP2A, PKA, and AMPK regulates HMG-CoA reductase.
- Glucagon (promotes phosphorylation)
- Insulin (promotes dephosphorylation)
- Intracellular cholesterol concentrations
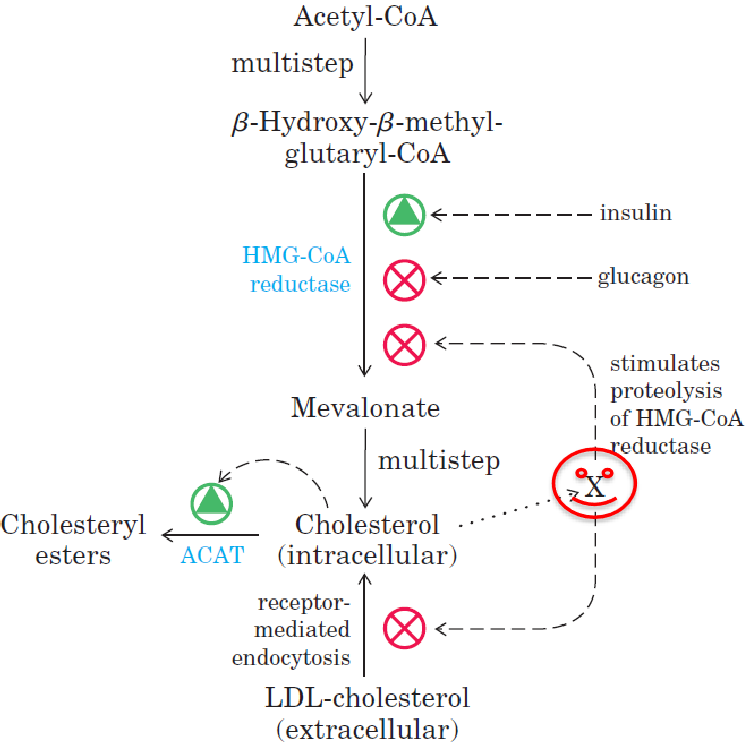
Figure 10.24: Regulating Cholesterol Metabolism