Introduction
The purpose of this site is to create a public, open-source repository for epidemiological research methods and reporting skills for observational studies. Epidemiology, the study of diseases and population health, is a broad field with ever-changing methods and often heated debates about proper designs, analyses, and approaches. Therefore, we think it is important that there is a place where epidemiologists can share their knowledge in an open and transparent manner. Drawing inspiration from the #epitwitter community, the Open Science Massive Online Course, and Data Methods, we hope that this will become a living site which can be used and modified by the epidemiology community and those interested in sharing epidemiological knowledge in accessible ways. For a quick peek into how this site works and how you can contribute, please check out the Frequently Asked Questions page.
This site is uses the R Bookdown package, which is built using R Markdown. All content is stored in the GitHub repository of Melissa Sharp, a doctoral student who worked on observational research methods. Please contact her if you have any comments, questions, or concerns.
Structure
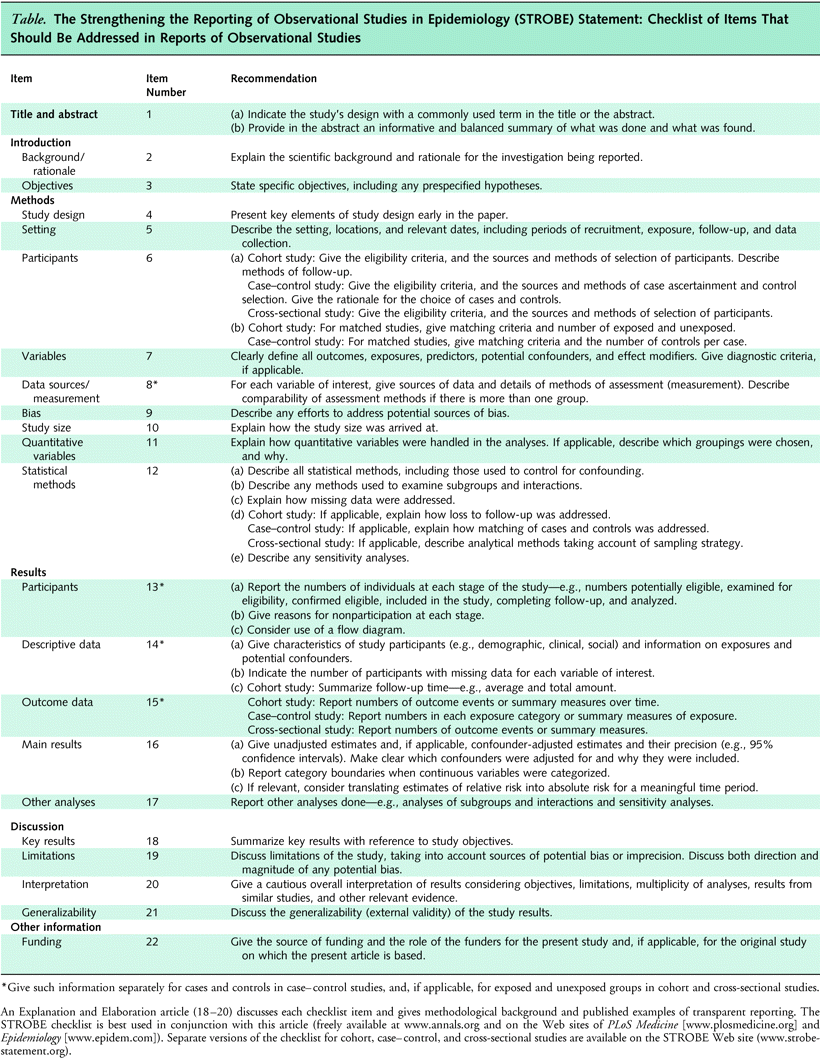
We aim to be as inclusive as possible but are structuring this site on the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guideline. STROBE was created in 2007 and is comprised of a 22-item checklist of essential items to report when discussing the results from a cohort, cross-sectional, or case-control study (Elm et al., 2007; Vandenbroucke et al., 2007). It has been endorsed by the International Commitee of Medcial Journal Editors and hundreds of journals and has spawned at least another 13 field- or method-specific extensions. (Benchimol et al., 2015; Cheng et al., 2016; Creinin & Chen, 2016 ; Field et al., 2014; Gallo et al., 2012; Horby et al., 2017; Hornell et al., 2017; Lachat et al., 2016; Little et al., 2009; O’Connor et al., 2016; Tacconelli et al., 2016; White et al., 2015; Zavada et al., 2014)
STROBE and this book follows the IMRaD (Introduction, Methods, Results, and Discussion) style of reporting research. The content from STROBE’s Explanation and Elaboration document (Vandenbroucke et al., 2007) will be included in this site as it explains why each item on the STROBE checklist is important and it gives examples of “good reporting”. Additional information will be supplied from the dissertation of Melissa Sharp and the projects contained within it – particularly results from a qualitative assessment of the content in the STROBE Extensions. (Sharp et al., 2018) Hopefully, over time, others will contribute additional examples and resources related to each STROBE item.
Content is designed in a modular format that is aligned with the design of the STROBE Checklist.
- If you are familiar with epidemiological research methods and STROBE, you can pick and choose what items to explore.
- Otherwise, if you are unexperienced, you can follow the structure in a more linear fashion, beginning with an introduction on the concept of reporting guidelines and their use.
Audience
The content in this book will appeal to those who work in observational research, need a refresher on certain epidemiological topics, or want to improve their scientific writing skills. Some baseline understanding of clinical or public health research may be helpful though since using STROBE should imply that you have or are working in health research.
Content
We hope to help you develop your understanding of study designs, data collection, statistical analysis, and interpretation of results. This site will not cover key aspects of the conduct of research, such as the skills needed for personal interactions with patients and participants, detailed analytical skills, regulatory frameworks, nor science communication skills needed to disseminate your work to the general public. All are important areas influencing items covered in this course but the breadth is too great to go over each area in depth. Rather, we aim to provide supplementary information along the way that will help you explore and grow in these areas if you so wish.
Funding Statement and Licensing
This site is a project of the Methods in Research on Research (MiRoR) project, supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 676207.The online version of this book is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Benchimol, E. I., Smeeth, L., Guttmann, A., Harron, K., Moher, D., Petersen, I., Sorensen, H. T., Elm, E. von, Langan, S. M., & Committee, R. W. (2015). The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLOS Medicine, 12(10), e1001885. https://doi.org/10.1371/journal.pmed.1001885
Cheng, A., Kessler, D., Mackinnon, R., Chang, T. P., Nadkarni, V. M., Hunt, E. A., Duval-Arnould, J., Lin, Y., Cook, D. A., Pusic, M., Hui, J., Moher, D., Egger, M., & Auerbach, M. (2016). Reporting guidelines for health care simulation research: Extensions to the CONSORT and STROBE statements. Advances in Simulation, 1, 25. https://doi.org/10.1186/s41077-016-0025-y
Elm, E. von, Altman, D. G., Egger, M., Pocock, S. J., Gotzsche, P. C., Vandenbroucke, J. P., & for the STROBE Initiative. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Annals of Internal Medicine, 147(8), 573. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Field, N., Cohen, T., Struelens, M. J., Palm, D., Cookson, B., Glynn, J. R., Gallo, V., Ramsay, M., Sonnenberg, P., MacCannell, D., Charlett, A., Egger, M., Green, J., Vineis, P., & Abubakar, I. (2014). Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An extension of the STROBE statement. The Lancet Infectious Diseases, 14(4), 341–352. https://doi.org/10.1016/S1473-3099(13)70324-4
Gallo, V., Egger, M., McCormack, V., Farmer, P. B., Ioannidis, J. P. A., Kirsch-Volders, M., Matullo, G., Phillips, D. H., Schoket, B., Stromberg, U., Vermeulen, R., Wild, C., Porta, M., & Vineis, P. (2012). STrengthening the Reporting of OBservational studies in Epidemiology Molecular Epidemiology (STROBE-ME): An extension of the STROBE statement. European Journal of Clinical Investigation, 42(1), 1–16. https://doi.org/10.1111/j.1365-2362.2011.02561.x
Horby, P. W., Laurie, K. L., Cowling, B. J., Engelhardt, O. G., Sturm-Ramirez, K., Sanchez, J. L., Katz, J. M., Uyeki, T. M., Wood, J., Van Kerkhove, M. D., & the CONSISE Steering Committee. (2017). CONSISE statement on the reporting of Seroepidemiologic Studies for influenza (ROSES-I statement): An extension of the STROBE statement. Influenza and Other Respiratory Viruses, 11(1), 2–14. https://doi.org/10.1111/irv.12411
Hornell, A., Berg, C., Forsum, E., Larsson, C., Sonestedt, E., Åkesson, A., Lachat, C., Hawwash, D., Kolsteren, P., Byrnes, G., Keyzer, W. D., Camp, J. V., Cade, J. E., Greenwood, D. C., Slimani, N., Cevallos, M., Egger, M., Huybrechts, I., & Wirfält, E. (2017). Perspective: An Extension of the STROBE Statement for Observational Studies in Nutritional Epidemiology (STROBE-nut): Explanation and Elaboration. Advances in Nutrition: An International Review Journal, 8(5), 652–678. https://doi.org/10.3945/an.117.015941
Lachat, C., Hawwash, D., Ocké, M. C., Berg, C., Forsum, E., Hörnell, A., Larsson, C., Sonestedt, E., Wirfält, E., Åkesson, A., Kolsteren, P., Byrnes, G., De Keyzer, W., Van Camp, J., Cade, J. E., Slimani, N., Cevallos, M., Egger, M., & Huybrechts, I. (2016). Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLOS Medicine, 13(6), e1002036. https://doi.org/10.1371/journal.pmed.1002036
Little, J., Higgins, J. P. T., Ioannidis, J. P. A., Moher, D., Gagnon, F., Elm, E. von, Khoury, M. J., Cohen, B., Davey-Smith, G., Grimshaw, J., Scheet, P., Gwinn, M., Williamson, R. E., Zou, G. Y., Hutchings, K., Johnson, C. Y., Tait, V., Wiens, M., Golding, J., … Birkett, N. (2009). STrengthening the REporting of Genetic Association Studies (STREGA)— An Extension of the STROBE Statement. PLOS Med, 6(2), e1000022. https://doi.org/10.1371/journal.pmed.1000022
O’Connor, A. M., Sargeant, J. M., Dohoo, I. R., Erb, H. N., Cevallos, M., Egger, M., Ersbøll, A. K., Martin, S. W., Nielsen, L. R., Pearl, D. L., Pfeiffer, D. U., Sanchez, J., Torrence, M. E., Vigre, H., Waldner, C., & Ward, M. P. (2016). Explanation and Elaboration Document for the STROBE-Vet Statement: Strengthening the Reporting of Observational Studies in Epidemiology – Veterinary Extension. Zoonoses and Public Health, 63(8), 662–698. https://doi.org/10.1111/zph.12315
Tacconelli, E., Cataldo, M. A., Paul, M., Leibovici, L., Kluytmans, J., Schröder, W., Foschi, F., Angelis, G. D., Waure, C. D., Cadeddu, C., Mutters, N. T., Gastmeier, P., & Cookson, B. (2016). STROBE-AMS: Recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open, 6(2), e010134. https://doi.org/10.1136/bmjopen-2015-010134
Vandenbroucke, J. P., Elm, E. von, Altman, D. G., Gotzsche, P. C., Mulrow, C. D., Pocock, S. J., Poole, C., Schlesselman, J. J., & Egger, M. (2007). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Epidemiology, 18(6), 805–835. https://doi.org/10.1097/EDE.0b013e3181577511
White, R. G., Hakim, A. J., Salganik, M. J., Spiller, M. W., Johnston, L. G., Kerr, L., Kendall, C., Drake, A., Wilson, D., Orroth, K., Egger, M., & Hladik, W. (2015). Strengthening the Reporting of Observational Studies in Epidemiology for respondent-driven sampling studies: "STROBE-RDS" statement. Journal of Clinical Epidemiology, 68(12), 1463–1471. https://doi.org/10.1016/j.jclinepi.2015.04.002
Zavada, J., Dixon, W. G., & Askling, J. (2014). Launch of a checklist for reporting longitudinal observational drug studies in rheumatology: A EULAR extension of STROBE guidelines based on experience from biologics registries. Annals of the Rheumatic Diseases, 73(3), 628. https://doi.org/http://dx.doi.org/10.1136/annrheumdis-2013-204102