8 Extending the Fundamental Principles of Moderation Analysis
As Hayes opened, “in this chapter, [we’ll see] how [the] principles of moderation analysis are applied when the moderator is dichotomous (rather than a continuum, as in the previous chapter) as well as when both focal antecedent and moderator are continuous (p. 267).”
8.1 Moderation with a dichotomous moderator
Here we load a couple necessary packages, load the data, and take a glimpse().
library(tidyverse)
library(brms)
disaster <- read_csv("data/disaster/disaster.csv")
glimpse(disaster)## Observations: 211
## Variables: 5
## $ id <dbl> 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23…
## $ frame <dbl> 1, 1, 1, 1, 1, 0, 0, 1, 0, 0, 1, 1, 0, 0, 1, 1, 1, 1, 0, 0, 1, 0, 1, 0, 1, 1, 0, …
## $ donate <dbl> 5.6, 4.2, 4.2, 4.6, 3.0, 5.0, 4.8, 6.0, 4.2, 4.4, 5.8, 6.2, 6.0, 4.2, 4.4, 5.8, 5…
## $ justify <dbl> 2.95, 2.85, 3.00, 3.30, 5.00, 3.20, 2.90, 1.40, 3.25, 3.55, 1.55, 1.60, 1.65, 2.6…
## $ skeptic <dbl> 1.8, 5.2, 3.2, 1.0, 7.6, 4.2, 4.2, 1.2, 1.8, 8.8, 1.0, 5.4, 2.2, 3.6, 7.8, 1.6, 1…Regardless of whether the antecedent variables are continuous or binary, the equation for the simple moderation is still
\[Y = i_Y + b_1 X + b_2 W + b_3 XW + e_Y.\]
We can use that equation to fit our first moderation model with a binary \(W\) (i.e., frame) like so.
model8.1 <-
brm(data = disaster,
family = gaussian,
justify ~ 1 + skeptic + frame + frame:skeptic,
cores = 4)Check the summary.
## Family: gaussian
## Links: mu = identity; sigma = identity
## Formula: justify ~ 1 + skeptic + frame + frame:skeptic
## Data: disaster (Number of observations: 211)
## Samples: 4 chains, each with iter = 2000; warmup = 1000; thin = 1;
## total post-warmup samples = 4000
##
## Population-Level Effects:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## Intercept 2.455 0.148 2.157 2.740 1.000 2727 3010
## skeptic 0.104 0.037 0.031 0.180 1.001 2631 2711
## frame -0.567 0.216 -0.986 -0.151 1.001 2332 2631
## skeptic:frame 0.202 0.054 0.096 0.310 1.001 2205 2528
##
## Family Specific Parameters:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## sigma 0.819 0.041 0.744 0.906 1.001 3270 2730
##
## Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
## is a crude measure of effective sample size, and Rhat is the potential
## scale reduction factor on split chains (at convergence, Rhat = 1).We’ll compute our Bayeisan \(R^2\) in the typical way.
## Estimate Est.Error Q2.5 Q97.5
## R2 0.249 0.044 0.164 0.333This model should look familiar to you because it is exactly the same model estimated in the analysis presented in Chapter 7 (see Table 7.4, model 3). The only differences between these two analyses are how the corresponding question is framed, meaning which variable is deemed the focal antecedent and which is the moderator, and how these variables are symbolically labeled as \(X\) and \(W\). In the analysis in Chapter 7, the focal antecedent variable was a dichotomous variable coding the framing of the cause of the disaster (labeled \(X\) then, but \(W\) now), whereas in this analysis, the focal antecedent is a continuous variable placing each person on a continuum of climate change skepticism (labeled \(W\) then, but \(X\) now), with the moderator being a dichotomous variable coding experimental condition.
So this example illustrates the symmetry property of interactions introduced in section 7.1. (p. 272)
8.1.1 Visualizing and probing the interaction.
For the plots in this chapter, we’ll take our color palette from the ochRe package, which provides Australia-inspired colors. We’ll also use a few theme settings from good-old ggthemes. As in the last chapter, we’ll save our adjusted theme settings as an object, theme_08.
library(ggthemes)
library(ochRe)
theme_08 <-
theme_minimal() +
theme(panel.grid.minor = element_blank(),
plot.background = element_rect(fill = ochre_palettes[["olsen_seq"]][8],
color = "transparent"))Happily, the ochRe package has a handy convenience function, viz_palette(), that makes it easy to preview the colors available in a given palette. We’ll be using “olsen_qual” and “olsen_seq”.
Behold our Figure 8.3.
# these will come in handy with `geom_text()`, below
green_slope <- (fixef(model8.1)["skeptic", 1] + fixef(model8.1)[4, 1]) %>% round(digits = 3)
blue_slope <- fixef(model8.1)["skeptic", 1] %>% round(digits = 3)
# define the new data
nd <-
crossing(frame = 0:1,
skeptic = c(0, 7))
# extract the `fitted()` draws
fitted(model8.1,
newdata = nd,
summary = F) %>%
data.frame() %>%
gather() %>%
# bind the new data
bind_cols(
nd %>%
expand(nesting(frame, skeptic),
iter = 1:4000)
) %>%
# plot!
ggplot(aes(x = skeptic, y = value,
group = interaction(frame, iter),
color = frame %>% as.character())) +
geom_line(aes(color = frame %>% as.character()),
size = 1/6, alpha = 1/25) +
geom_text(data = tibble(skeptic = c(4, 4.6),
value = c(3.5, 2.6),
frame = 1:0,
iter = 0,
label = c(paste("the slope when frame = 1 is about", green_slope),
paste("the slope when frame = 0 is about", blue_slope)),
angle = c(28, 5)),
aes(label = label, angle = angle)) +
scale_color_manual(NULL, values = ochre_palettes[["olsen_qual"]][(5:6)]) +
scale_x_continuous(breaks = 1:6) +
coord_cartesian(xlim = 1:6,
ylim = 2:4) +
labs(title = "The moderation effect in a spaghetti plot",
subtitle = "Using brms defaults, we have 4000 posterior draws. Much like we have\n4000 draws for each model parameter, when we combine those draws\nacross parameters, we end up with 4000 model equations. The\nconsequence is we can also express the line plot as 4000\nsemitransparent lines, one for each level of our moderator, frame.",
x = expression(paste("Climate Change Skepticism (", italic("X"), ")")),
y = "Strength of Justification for Withholding Aid") +
theme_08 +
theme(legend.position = "none")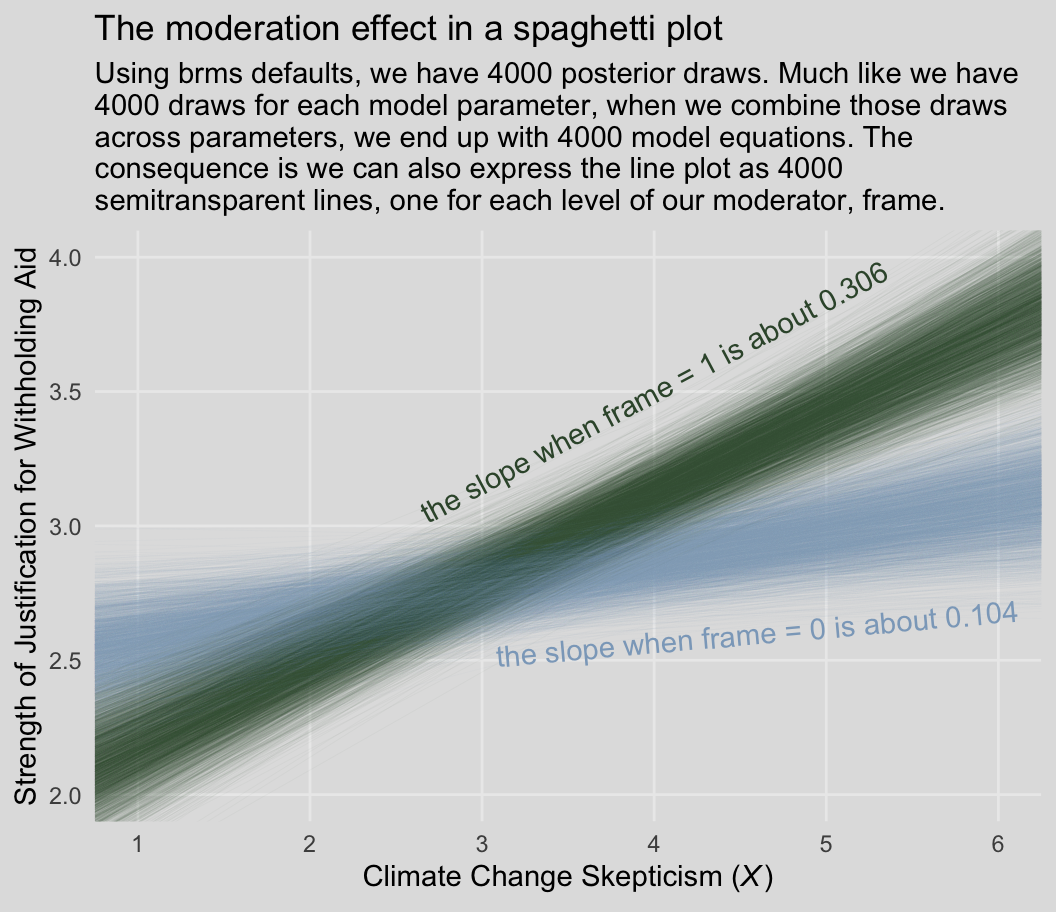
In addition to our fancy Australia-inspired colors, we’ll also play around a bit with spaghetti plots in this chapter. To my knowledge, this use of spaghetti plots is uniquely Bayesian. If you’re trying to wrap your head around what on earth we just did, take a look at the first few rows from posterior_samples() object, post.
## b_Intercept b_skeptic b_frame b_skeptic:frame sigma lp__
## 1 2.171268 0.18122884 -0.6213774 0.1814370 0.8144831 -264.4922
## 2 2.112918 0.19286032 -0.5346854 0.1749145 0.8169612 -264.5730
## 3 2.580092 0.04726581 -0.3385162 0.1657682 0.8691147 -264.6696
## 4 2.609206 0.04635323 -0.3941262 0.1594145 0.8658938 -264.7612
## 5 2.408386 0.12717550 -0.5910781 0.1956912 0.8121655 -260.1720
## 6 2.383672 0.11459057 -0.5955617 0.2302462 0.8024598 -260.5167The head() function returned six rows, each one corresponding to the credible parameter values from a given posterior draw. The lp__ is uniquely Bayesian and beyond the scope of this project. You might think of sigma as the Bayesian analogue to what the OLS folks often refer to as error or the residual variance. Hayes doesn’t tend to emphasize it in this text, but it’s something you’ll want to pay increasing attention to as you move along in your Bayesian career. All the columns starting with b_ are the regression parameters, the model coefficients or the fixed effects. But anyways, notice that those b_ columns correspond to the four parameter values in Formula 8.2 on page 270. Here they are, but reformatted to more closely mimic the text:
- \(\hat{Y}\) = 2.171 + 0.181\(X\) + -0.621\(W\) + 0.181XW
- \(\hat{Y}\) = 2.113 + 0.193\(X\) + -0.535\(W\) + 0.175XW
- \(\hat{Y}\) = 2.58 + 0.047\(X\) + -0.339\(W\) + 0.166XW
- \(\hat{Y}\) = 2.609 + 0.046\(X\) + -0.394\(W\) + 0.159XW
- \(\hat{Y}\) = 2.408 + 0.127\(X\) + -0.591\(W\) + 0.196XW
- \(\hat{Y}\) = 2.384 + 0.115\(X\) + -0.596\(W\) + 0.23XW
Each row of post, each iteration or posterior draw, yields a full model equation that is a credible description of the data–or at least as credible as we can get within the limits of the model we have specified, our priors (which we typically cop out on and just use defaults in this project), and how well those fit when applied to the data at hand. So when we use brms convenience functions like fitted(), we pass specific predictor values through those 4000 unique model equations, which returns 4000 similar but distinct expected \(Y\)-values. So although a nice way to summarize those 4000 values is with summaries such as the posterior mean/median and 95% intervals, another way is to just plot an individual regression line for each of the iterations. That is what’s going on when we depict out models with a spaghetti plot.
The thing I like about spaghetti plots is that they give a three-dimensional sense of the posterior. Note that each individual line is very skinny and semitransparent. When you pile a whole bunch of them atop each other, the peaked or most credible regions of the posterior are the most saturated in color. Less credible posterior regions almost seamlessly merge into the background. Also, note how the combination of many similar but distinct straight lines results in a bowtie shape. Hopefully this clarifies where that shape’s been coming from when we use geom_ribbon() to plot the 95% intervals.
Back to the text, on the bottom of page 274, Hayes pointed out the conditional effect of skeptic when frame == 1 is \(b_1 + b_3 = 0.306\). We can show that with a little arithmetic followed up with tidybayes::mean_qi().
library(tidybayes)
post <-
post %>%
mutate(`b1 + b3` = b_skeptic + `b_skeptic:frame`)
post %>%
mean_qi(`b1 + b3`) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 1 x 6
## `b1 + b3` .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 0.306 0.23 0.384 0.95 mean qiBut anyways, you could recode frame in a number of ways, including if_else() or, in this case, by simple arithmetic.
With frame_ep in hand, we’re ready to refit the model.
model8.2 <-
update(model8.1,
newdata = disaster,
formula = justify ~ 1 + skeptic + frame_ep + frame_ep:skeptic,
cores = 4)## Family: gaussian
## Links: mu = identity; sigma = identity
## Formula: justify ~ skeptic + frame_ep + skeptic:frame_ep
## Data: disaster (Number of observations: 211)
## Samples: 4 chains, each with iter = 2000; warmup = 1000; thin = 1;
## total post-warmup samples = 4000
##
## Population-Level Effects:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## Intercept 1.88 0.16 1.56 2.19 1.00 2193 2986
## skeptic 0.31 0.04 0.23 0.39 1.00 2194 2480
## frame_ep 0.57 0.21 0.14 0.98 1.00 1891 2273
## skeptic:frame_ep -0.20 0.05 -0.31 -0.10 1.00 1907 2081
##
## Family Specific Parameters:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## sigma 0.82 0.04 0.74 0.90 1.00 3017 2732
##
## Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
## is a crude measure of effective sample size, and Rhat is the potential
## scale reduction factor on split chains (at convergence, Rhat = 1).Our results match nicely with the formula on page 275.
If you want to follow along with Hayes on page 276 and isolate the 95% credible intervals for the skeptic parameter, you can use the posterior_interval() function.
## 2.5% 97.5%
## 0.231 0.3898.2 Interaction between two quantitative variables
Here’s the glbwarm data.
## Observations: 815
## Variables: 7
## $ govact <dbl> 3.6, 5.0, 6.6, 1.0, 4.0, 7.0, 6.8, 5.6, 6.0, 2.6, 1.4, 5.6, 7.0, 3.8, 3.4, 4.2, …
## $ posemot <dbl> 3.67, 2.00, 2.33, 5.00, 2.33, 1.00, 2.33, 4.00, 5.00, 5.00, 1.00, 4.00, 1.00, 5.…
## $ negemot <dbl> 4.67, 2.33, 3.67, 5.00, 1.67, 6.00, 4.00, 5.33, 6.00, 2.00, 1.00, 4.00, 5.00, 4.…
## $ ideology <dbl> 6, 2, 1, 1, 4, 3, 4, 5, 4, 7, 6, 4, 2, 4, 5, 2, 6, 4, 2, 4, 4, 2, 6, 4, 4, 3, 4,…
## $ age <dbl> 61, 55, 85, 59, 22, 34, 47, 65, 50, 60, 71, 60, 71, 59, 32, 36, 69, 70, 41, 48, …
## $ sex <dbl> 0, 0, 1, 0, 1, 0, 1, 1, 1, 1, 1, 0, 1, 0, 1, 1, 1, 0, 0, 0, 0, 1, 1, 1, 1, 1, 1,…
## $ partyid <dbl> 2, 1, 1, 1, 1, 2, 1, 1, 2, 3, 2, 1, 1, 1, 1, 1, 2, 3, 1, 3, 2, 1, 3, 2, 1, 1, 1,…In this section we add three covariates (i.e., \(C\) variables) to the basic moderation model. Although Hayes made a distinction between the \(X\), \(M\), and \(C\) variables in the text, that distinction is conceptual and doesn’t impact the way we enter them into brm(). Rather, the brm() formula clarifies they’re all just predictors.
model8.3 <-
brm(data = glbwarm,
family = gaussian,
govact ~ 1 + negemot + age + negemot:age + posemot + ideology + sex,
cores = 4)Our results cohere nicely with the Hayes’s formula in the middle of page 278 or with the results he displayed in Table 8.2.
## Family: gaussian
## Links: mu = identity; sigma = identity
## Formula: govact ~ 1 + negemot + age + negemot:age + posemot + ideology + sex
## Data: glbwarm (Number of observations: 815)
## Samples: 4 chains, each with iter = 2000; warmup = 1000; thin = 1;
## total post-warmup samples = 4000
##
## Population-Level Effects:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## Intercept 5.176 0.340 4.528 5.838 1.000 2494 2796
## negemot 0.119 0.083 -0.042 0.282 1.002 2320 2502
## age -0.024 0.006 -0.036 -0.012 1.001 2279 2394
## posemot -0.021 0.028 -0.078 0.034 1.002 4074 2772
## ideology -0.211 0.027 -0.264 -0.159 1.000 4268 2995
## sex -0.012 0.075 -0.163 0.134 1.002 4037 2925
## negemot:age 0.006 0.002 0.003 0.009 1.001 2364 2564
##
## Family Specific Parameters:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## sigma 1.059 0.026 1.009 1.112 1.000 3783 2748
##
## Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
## is a crude measure of effective sample size, and Rhat is the potential
## scale reduction factor on split chains (at convergence, Rhat = 1).Here’s the \(R^2\) summary.
## Estimate Est.Error Q2.5 Q97.5
## R2 0.401 0.02 0.36 0.44As the \(R^2\) is a good bit away from the boundaries, it’s nicely Gaussian.
bayes_R2(model8.3, summary = F) %>%
as_tibble() %>%
ggplot(aes(x = R2)) +
geom_density(size = 0, fill = ochre_palettes[["olsen_qual"]][(1)]) +
scale_y_continuous(NULL, breaks = NULL) +
coord_cartesian(xlim = 0:1) +
labs(title = expression(paste("Loot at how Gaussian our ", italic(R)^2, " is!")),
subtitle = expression(paste("No need to put a ", italic(p), "-value on that sucker.")),
x = NULL) +
theme_08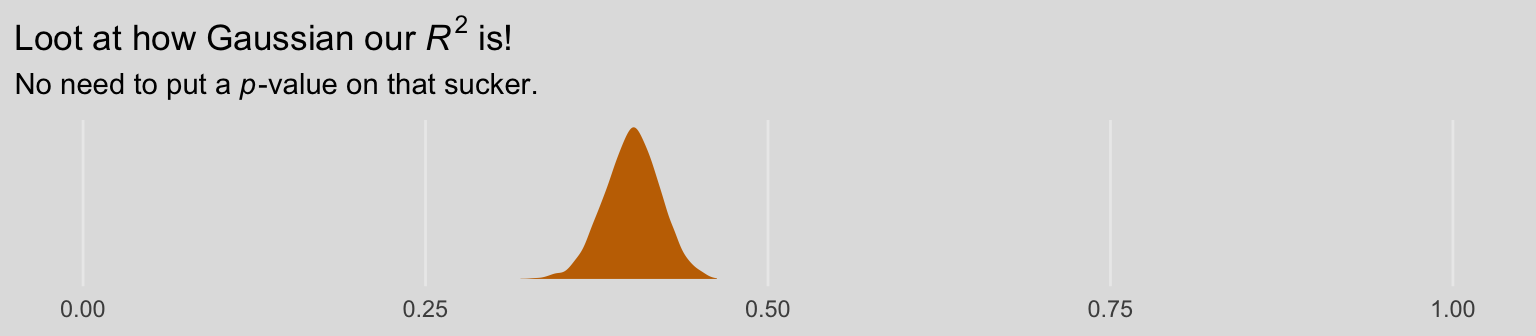
8.2.1 Visualizing and probing the interaction.
For our version of Figure 8.5, we’ll need to adjust our nd data for fitted().
(
nd <-
crossing(negemot = c(1, 6),
age = c(30, 50, 70)) %>%
mutate(posemot = mean(glbwarm$posemot),
ideology = mean(glbwarm$ideology),
sex = mean(glbwarm$sex))
)## # A tibble: 6 x 5
## negemot age posemot ideology sex
## <dbl> <dbl> <dbl> <dbl> <dbl>
## 1 1 30 3.13 4.08 0.488
## 2 1 50 3.13 4.08 0.488
## 3 1 70 3.13 4.08 0.488
## 4 6 30 3.13 4.08 0.488
## 5 6 50 3.13 4.08 0.488
## 6 6 70 3.13 4.08 0.488Our fitted() and ggplot2 code will be quite similar to the last spaghetti plot. Only this time we’ll use filter() to reduce the number of posterior draws we show in the plot.
# these will come in handy with `geom_text()`, below
slope_30 <- (fixef(model8.3)["negemot", 1] + fixef(model8.3)["negemot:age", 1] * 30) %>% round(digits = 3)
slope_50 <- (fixef(model8.3)["negemot", 1] + fixef(model8.3)["negemot:age", 1] * 50) %>% round(digits = 3)
slope_70 <- (fixef(model8.3)["negemot", 1] + fixef(model8.3)["negemot:age", 1] * 70) %>% round(digits = 3)
fitted(model8.3, newdata = nd,
summary = F) %>%
data.frame() %>%
gather() %>%
bind_cols(
nd %>%
expand(nesting(negemot, age),
iter = 1:4000)
) %>%
filter(iter <= 100) %>%
ggplot(aes(x = negemot, y = value,
group = interaction(age, iter),
color = age %>% as.character())) +
geom_line(aes(color = age %>% as.character()),
size = 3/4, alpha = 1/8) +
geom_text(data = tibble(negemot = 6.1,
value = c(5.4, 5.7, 6),
age = c(30, 50, 70),
iter = 0,
label = c(paste("the slope for 30 year olds is about", slope_30),
paste("the slope for 50 year olds is about", slope_50),
paste("the slope for 70 year olds is about", slope_70))),
aes(label = label),
hjust = 0) +
scale_color_manual(NULL, values = ochre_palettes[["olsen_qual"]][c(4, 1, 3)]) +
scale_x_continuous(breaks = 1:6, limits = c(1, 9)) +
scale_y_continuous("Support for Government Action", limits = c(2.9, 6.1)) +
labs(title = "The moderation effect of age on negemot",
x = expression(paste("Negative Emotions about Climate Change (", italic("X"), ")"))) +
theme_08 +
theme(legend.position = "none")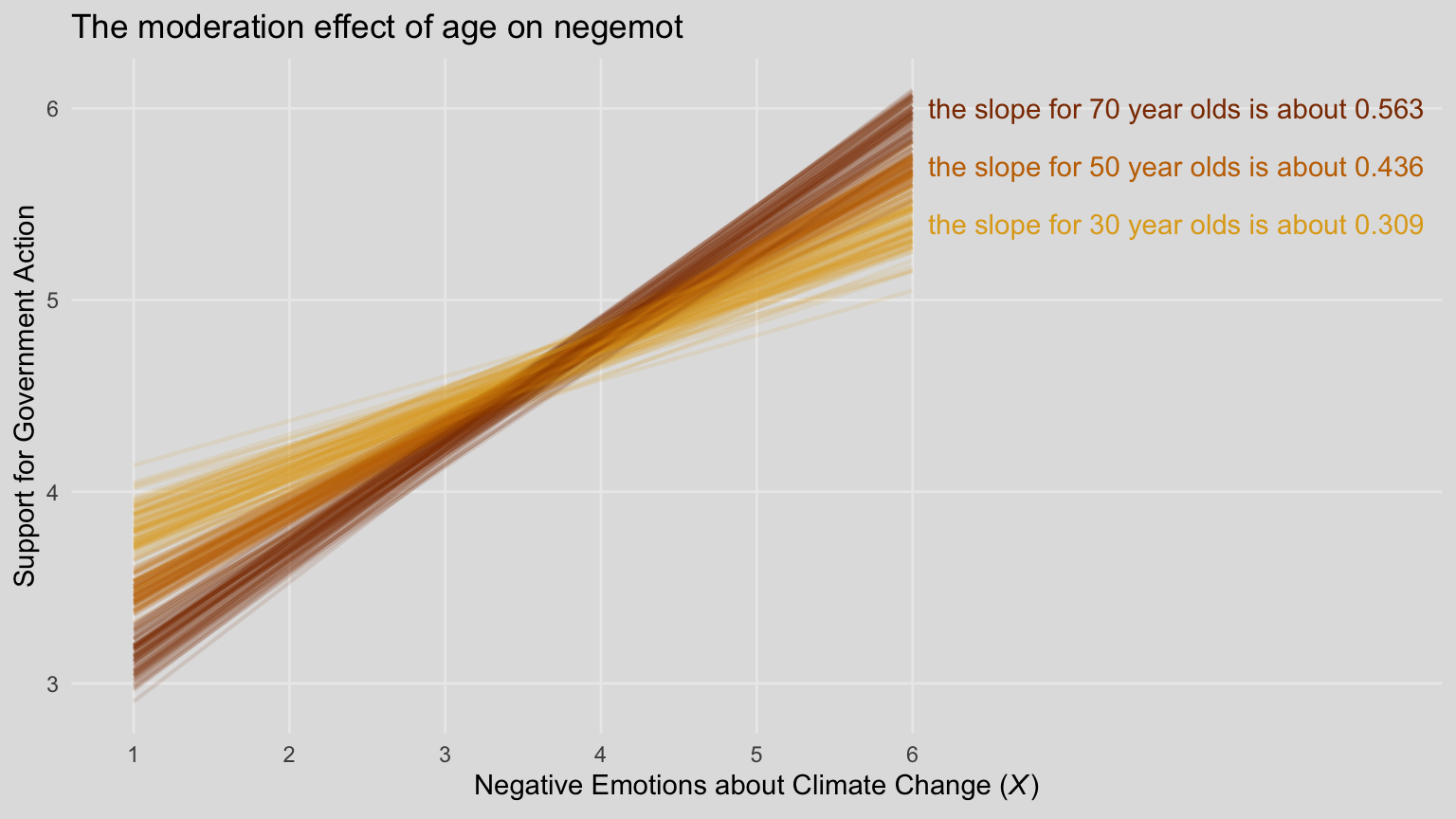
When we reduce the number of lines depicted in the plot, we lose some of the three-dimensional illusion. It’s nice, however, to get a closer look to each individual line. To each their own.
We’ll continue with our spaghetti plot approach for Figure 8.7. Again, when we made the JN technique plot for Chapter 7, we computed values for the posterior mean and the 95% intervals. Because the intervals follow a bowtie shape, we had to compute the \(Y\)-values for many values across the x-axis in order to make the curve look smooth. But as long as we stick with the spaghetti plot approach, all we need are the values at the endpoints of each iteration. Although each line is straight, the combination of many lines is what produces the bowtie effect.
# here is our primary data object
post <-
posterior_samples(model8.3) %>%
transmute(at_15 = b_negemot + `b_negemot:age` * 15,
at_90 = b_negemot + `b_negemot:age` * 90,
iter = 1:n()) %>%
pivot_longer(-iter,
names_to = "age") %>%
mutate(age = str_remove(age, "at_") %>% as.double())
# here we compute the points for the posterior mean
post_means <-
post %>%
group_by(age) %>%
summarize(value = mean(value)) %>%
mutate(iter = 0)
# plot!
post %>%
filter(iter < 501) %>%
ggplot(aes(x = age, y = value, group = iter)) +
geom_line(color = ochre_palettes[["olsen_qual"]][1],
alpha = 1/12) +
geom_line(data = post_means,
color = ochre_palettes[["olsen_qual"]][3],
size = 1.1) +
scale_y_continuous(breaks = seq(from = -.25, to = 1, by = .25)) +
coord_cartesian(xlim = c(20, 85),
ylim = c(-.25, 1)) +
labs(subtitle = "Each orange line is the consequence of one of 1000 posterior draws. All are\ncredible regression lines for the data, but because they are semitransparent,\nthe most credible regions are the ones with the darkest color. The line\ncorresponding to the posterior mean is in red-orange.",
x = expression(paste("Age (", italic("W"), ")")),
y = "Conditional Effects of Negative Emotions on\nSupport for Government Action") +
theme_08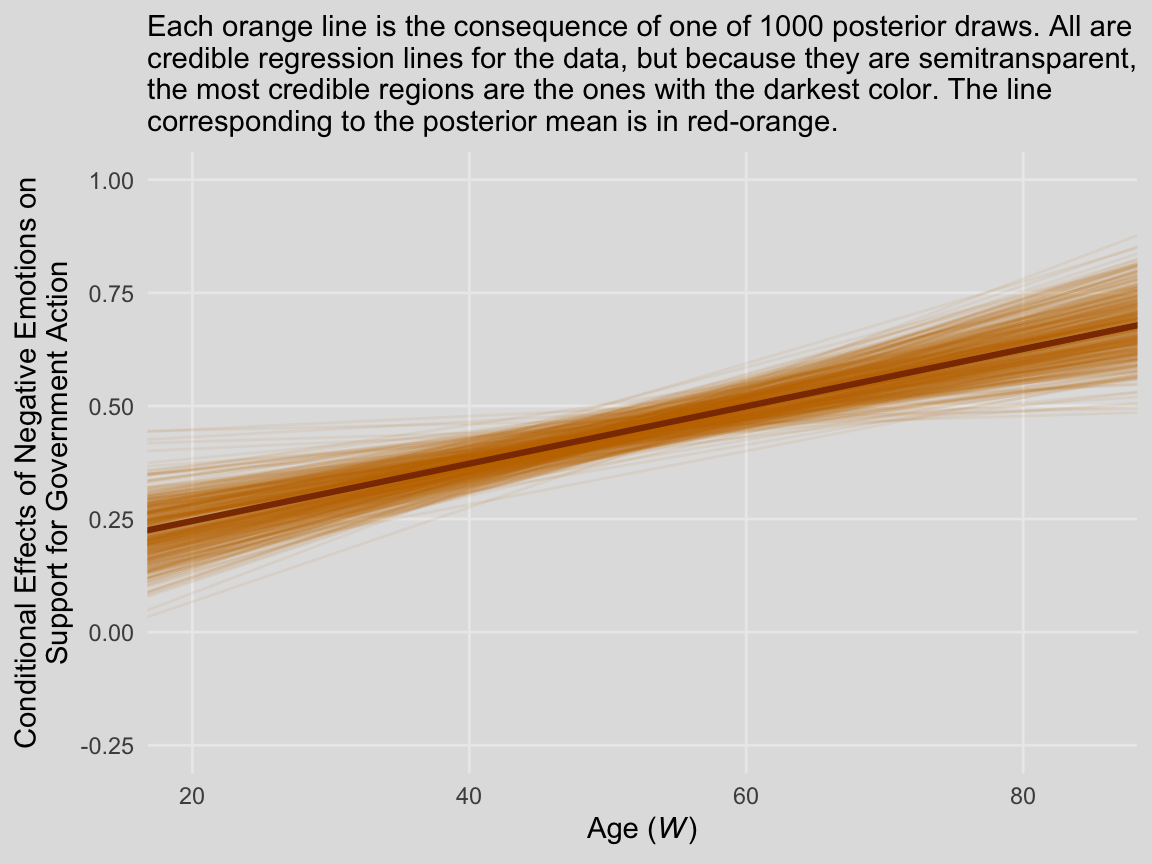
In other words, each of those orange lines is a credible expression of \(\theta_{X \rightarrow Y}\) (i.e., \(b_1 + b_3 W\)) across a continuous range of \(W\) values.
8.3 Hierarchical versus simultaneous entry
Many investigators test a moderation hypothesis in regression analysis using a method that on the surface seems different than the procedure described thus far. This alternative approach is to build a regression model by adding the product of \(X\) and \(W\) to a model already containing \(X\) and \(W\). This procedure is sometimes called hierarchical regression or hierarchical variable entry (and easily confused by name with hierarchical linear modeling, which is an entirely different thing). The goal using this method is to determine whether allowing \(X\)’s effect to be contingent on \(W\) produces a better fitting model than one in which the effect of \(X\) is constrained to be unconditional on \(W\). According to the logic of hierarchical entry, if the contingent model accounts for more of the variation in Y than the model that forces \(X\)’s effect to be independent of \(W\), then the better model is one in which \(W\) is allowed to moderate \(X\)’s effect. Although this approach works, it is a widely believed myth that it is necessary to use this approach in order to test a moderation hypothesis. (p. 289, emphasis in the original)
Although this method is not necessary, it can be handy to slowly build your model. This method can also serve nice rhetorical purposes in a paper. Anyway, here’s our multivariable but non-moderation model, model8.4.
Here we’ll compute the corresponding \(R^2\) and compare it with the one for the original interaction model with a difference score.
# the moderation model's R2
r2 <-
bayes_R2(model8.1, summary = F) %>%
data.frame() %>%
set_names("moderation_model") %>%
# here we add the multivaraible model's R2
bind_cols(
bayes_R2(model8.4, summary = F) %>%
data.frame() %>%
set_names("multivariable_model")
) %>%
# we'll need a difference score
mutate(difference = moderation_model - multivariable_model) %>%
# putting the data in the long format and grouping will make summarizing easier
pivot_longer(everything(),
names_to = "R2")
r2 %>%
group_by(R2) %>%
median_qi(value) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 3 x 7
## R2 value .lower .upper .width .point .interval
## <chr> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 difference 0.05 -0.073 0.169 0.95 median qi
## 2 moderation_model 0.25 0.164 0.333 0.95 median qi
## 3 multivariable_model 0.2 0.113 0.284 0.95 median qiNote that the Bayesian \(R^2\) performed differently than the \(F\)-test in the text.
r2 %>%
filter(R2 == "difference") %>%
ggplot(aes(x = value, fill = model)) +
geom_density(size = 0, fill = ochre_palettes[["olsen_seq"]][14]) +
scale_x_continuous(NULL, limits = c(-.4, .4)) +
scale_y_continuous(NULL, breaks = NULL) +
labs(title = expression(paste("The Bayesian ", Delta, italic(R)^2, " distribution")),
subtitle = "Although most of the posterior mass is positive--suggesting the moderation model accounted for more variance than\nthe simple multivariable model--, a substantial portion of the postrior is within the negative parameter space. Sure,\nif we had to bet, the safer bet is on the moderation model. But that bet wouled be quite uncertain and we might well\nloose our shirts. Also, note the width of the distribution; credible values range from -0.1 to nearly 0.2.") +
theme_08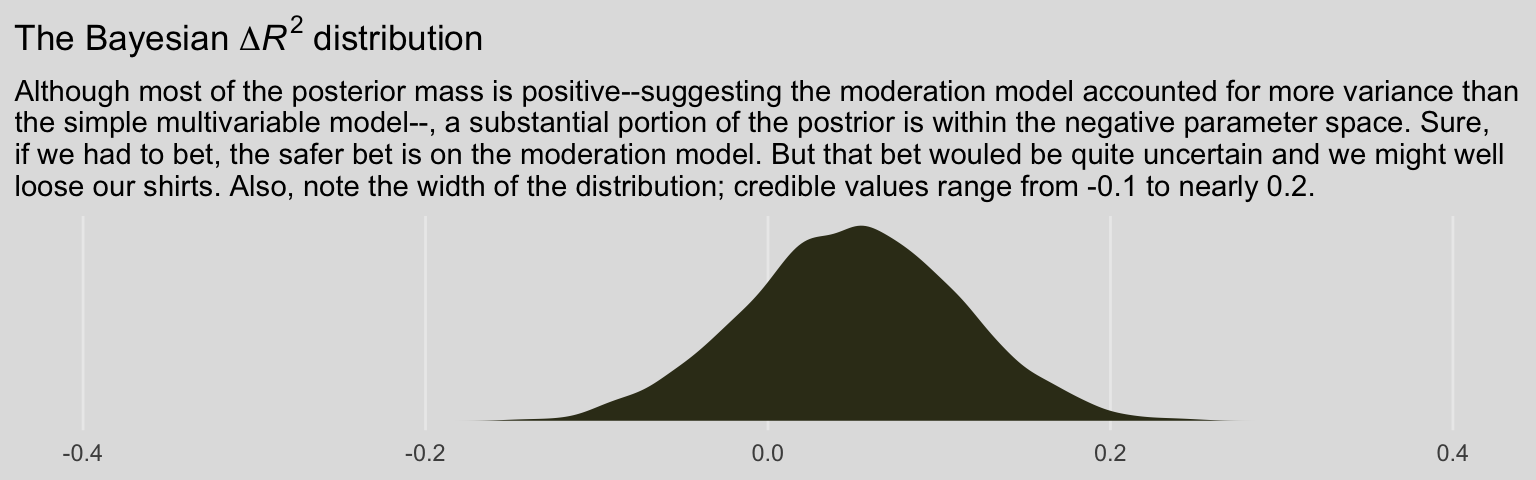
We can also compare these with the LOO, which, as is typical of information criteria, corrects for model complexity. First, we compute them and attach the results to the model fit objects.
Now use the loo_compare() function to compare them directly.
## elpd_diff se_diff elpd_loo se_elpd_loo p_loo se_p_loo looic se_looic
## model8.1 0.0 0.0 -259.1 10.8 5.5 0.9 518.2 21.6
## model8.4 -5.8 4.1 -264.8 11.2 4.9 0.9 529.7 22.4As a reminder, we generally prefer models with lower information criteria, which in this case is clearly the moderation model (i.e., model8.1). However, the standard error value (i.e., se_diff) for the difference (i.e., elpd_diff) is quite large, which suggests that the model with the lowest value isn’t the clear winner. Happily, these results match nicely with the Bayesian \(R^2\) difference score. The moderation model appears somewhat better than the multivariable model, but its superiority is hardly decisive.
8.4 The equivalence between moderated regression analysis and a 2 X 2 factorial analysis of variance
I’m just not going to encourage ANOVA \(F\)-testing methodology. However, I will show the Bayesian regression model. First, here are the data.
## Observations: 541
## Variables: 7
## $ policy <dbl> 1, 0, 1, 1, 1, 0, 1, 1, 0, 1, 1, 1, 0, 1, 0, 1, 0, 0, 1, 1, 0, 1, 0, 1, 0, 1, 0,…
## $ interest <dbl> 4.0, 2.0, 3.0, 1.0, 1.0, 2.0, 1.0, 2.5, 3.0, 1.0, 2.0, 3.5, 1.0, 1.0, 1.5, 3.0, …
## $ age <dbl> 39, 57, 63, 56, 50, 87, 33, 64, 82, 28, 18, 52, 42, 39, 64, 72, 54, 84, 55, 27, …
## $ educ <dbl> 3, 3, 2, 5, 3, 2, 7, 2, 3, 3, 1, 1, 5, 4, 3, 2, 3, 4, 7, 2, 3, 5, 4, 5, 5, 3, 3,…
## $ male <dbl> 1, 1, 0, 1, 0, 1, 0, 1, 0, 0, 1, 0, 0, 1, 0, 0, 0, 0, 1, 1, 1, 0, 1, 1, 1, 1, 1,…
## $ conserv <dbl> 4, 3, 6, 3, 3, 5, 6, 3, 6, 7, 4, 2, 7, 6, 5, 6, 6, 3, 7, 6, 5, 5, 3, 4, 6, 2, 7,…
## $ kerry <dbl> 1, 1, 1, 1, 1, 0, 0, 1, 0, 0, 0, 1, 1, 0, 1, 1, 1, 1, 0, 0, 0, 0, 1, 0, 0, 0, 0,…Fit the moderation model.
model8.5 <-
brm(data = caskets,
family = gaussian,
interest ~ 1 + policy + kerry + policy:kerry,
cores = 4)## Family: gaussian
## Links: mu = identity; sigma = identity
## Formula: interest ~ 1 + policy + kerry + policy:kerry
## Data: caskets (Number of observations: 541)
## Samples: 4 chains, each with iter = 2000; warmup = 1000; thin = 1;
## total post-warmup samples = 4000
##
## Population-Level Effects:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## Intercept 1.78 0.09 1.61 1.96 1.00 2374 2968
## policy -0.38 0.12 -0.63 -0.14 1.00 2096 2639
## kerry 0.60 0.13 0.34 0.84 1.00 2176 2434
## policy:kerry 0.36 0.17 0.03 0.71 1.00 1926 2375
##
## Family Specific Parameters:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## sigma 1.04 0.03 0.98 1.10 1.00 3741 2853
##
## Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
## is a crude measure of effective sample size, and Rhat is the potential
## scale reduction factor on split chains (at convergence, Rhat = 1).Those results don’t look anything like what Hayes reported in Tables 8.3 or 8.4. However, a little deft manipulation of the posterior samples can yield equivalent results to Hayes’s Table 8.3.
post <-
posterior_samples(model8.5) %>%
mutate(Y_bar_1 = b_Intercept + b_policy * 0 + b_kerry * 0 + `b_policy:kerry` * 0 * 0,
Y_bar_3 = b_Intercept + b_policy * 0 + b_kerry * 1 + `b_policy:kerry` * 0 * 1,
Y_bar_2 = b_Intercept + b_policy * 1 + b_kerry * 0 + `b_policy:kerry` * 1 * 0,
Y_bar_4 = b_Intercept + b_policy * 1 + b_kerry * 1 + `b_policy:kerry` * 1 * 1,
Y_bar_12 = b_Intercept + b_policy * .5 + b_kerry * 0 + `b_policy:kerry` * .5 * 0,
Y_bar_34 = b_Intercept + b_policy * .5 + b_kerry * 1 + `b_policy:kerry` * .5 * 1,
Y_bar_13 = b_Intercept + b_policy * 0 + b_kerry * .5 + `b_policy:kerry` * 0 * .5,
Y_bar_24 = b_Intercept + b_policy * 1 + b_kerry * .5 + `b_policy:kerry` * 1 * .5)Here are the cell-specific means in Table 8.3.
post %>%
pivot_longer(Y_bar_1:Y_bar_4) %>%
group_by(name) %>%
median_qi(value) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 4 x 7
## name value .lower .upper .width .point .interval
## <chr> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 Y_bar_1 1.78 1.61 1.96 0.95 median qi
## 2 Y_bar_2 1.40 1.23 1.58 0.95 median qi
## 3 Y_bar_3 2.38 2.21 2.56 0.95 median qi
## 4 Y_bar_4 2.36 2.19 2.53 0.95 median qiAnd here are the marginal means from Table 8.3.
post %>%
pivot_longer(Y_bar_12:Y_bar_24) %>%
group_by(name) %>%
median_qi(value) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 4 x 7
## name value .lower .upper .width .point .interval
## <chr> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 Y_bar_12 1.59 1.47 1.72 0.95 median qi
## 2 Y_bar_13 2.08 1.96 2.21 0.95 median qi
## 3 Y_bar_24 1.88 1.76 2.00 0.95 median qi
## 4 Y_bar_34 2.37 2.24 2.50 0.95 median qiFor kicks and giggles, here are what the cell-specific means look like in box plots.
post %>%
pivot_longer(Y_bar_1:Y_bar_4) %>%
ggplot(aes(x = name, y = value, fill = name)) +
geom_boxplot(size = 1/3) +
scale_fill_manual(values = ochre_palettes[["olsen_qual"]][c(5, 6, 4, 3)]) +
labs(title = "Cell-specific effects",
x = NULL,
y = "interest") +
theme_08 +
theme(legend.position = "none")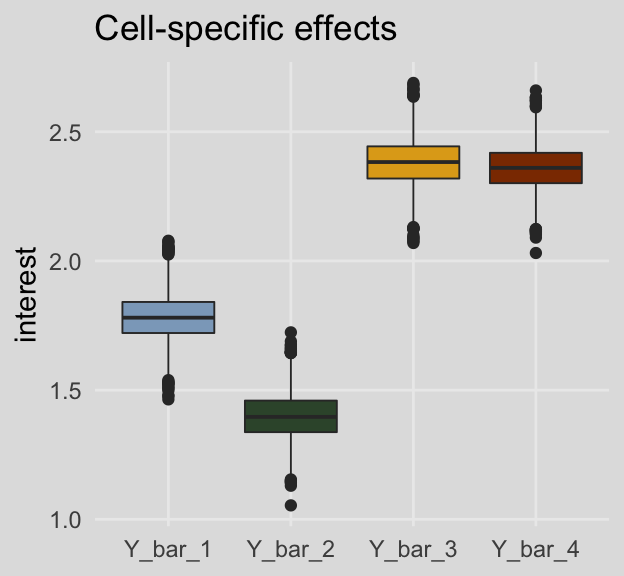
And here are the same for the marginal means. This time we’ll show the shapes of the posteriors with violin plots with horizontal lines depicting the median and interquartile ranges.
post %>%
pivot_longer(Y_bar_12:Y_bar_24) %>%
ggplot(aes(x = name, y = value, fill = name)) +
geom_violin(draw_quantiles = c(.25, .5, .75),
color = ochre_palettes[["olsen_seq"]][8]) +
scale_fill_manual(values = ochre_palettes[["olsen_qual"]][c(5, 6, 4, 3)]) +
labs(title = "Marginal means",
x = NULL,
y = "interest") +
theme_08 +
theme(legend.position = "none")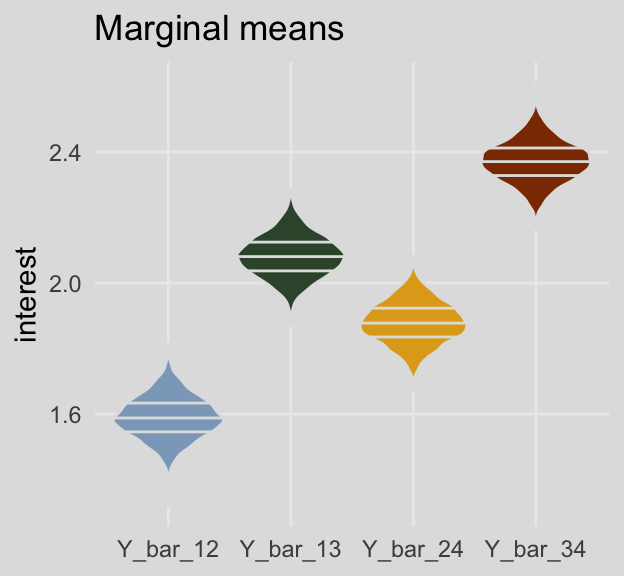
On page 294, Hayes used point estimates to compute the simple effect of policy information among Kerry supporters and then the same thing among Bush supporters. Here’s how we’d do that when working with the full vector of posterior draws.
post %>%
mutate(simple_effect_Kerry = Y_bar_4 - Y_bar_3,
simple_effect_Bush = Y_bar_2 - Y_bar_1) %>%
pivot_longer(contains("simple")) %>%
group_by(name) %>%
mean_qi(value) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 7
## name value .lower .upper .width .point .interval
## <chr> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 simple_effect_Bush -0.382 -0.631 -0.142 0.95 mean qi
## 2 simple_effect_Kerry -0.023 -0.265 0.221 0.95 mean qiSo then computing the main effect for policy information using the simple effects is little more than an extension of those steps.
post %>%
transmute(main_effect = ((Y_bar_4 - Y_bar_3) + (Y_bar_2 - Y_bar_1))/2) %>%
mean_qi(main_effect) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 1 x 6
## main_effect .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 -0.203 -0.381 -0.029 0.95 mean qiAnd we get the same results by strategically subtracting the marginal means.
post %>%
transmute(main_effect = Y_bar_24 - Y_bar_13) %>%
mean_qi(main_effect) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 1 x 6
## main_effect .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 -0.203 -0.381 -0.029 0.95 mean qiThe main effect of for candidate is similarly computed using either approach.
post %>%
transmute(main_effect = ((Y_bar_4 - Y_bar_2) + (Y_bar_3 - Y_bar_1))/2) %>%
mean_qi(main_effect) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 1 x 6
## main_effect .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 0.78 0.606 0.958 0.95 mean qipost %>%
transmute(main_effect = Y_bar_34 - Y_bar_12) %>%
mean_qi(main_effect) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 1 x 6
## main_effect .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 0.78 0.606 0.958 0.95 mean qiWe don’t have an \(F\)-test for our Bayesian moderation model. But we do have an interaction term. Here’s its distribution.
post %>%
ggplot(aes(x = `b_policy:kerry`)) +
geom_density(size = 0,
fill = ochre_palettes[["olsen_qual"]][2]) +
geom_vline(xintercept = fixef(model8.5)["policy:kerry", c(1, 3, 4)],
color = ochre_palettes[["olsen_seq"]][8], linetype = c(1, 2, 2)) +
scale_x_continuous(NULL,
breaks = fixef(model8.5)["policy:kerry", c(1, 3, 4)],
labels = fixef(model8.5)["policy:kerry", c(1, 3, 4)] %>% round(digits = 2)) +
scale_y_continuous(NULL, breaks = NULL) +
labs(title = "The interaction term, `policy:kerry`",
subtitle = "The solid vertical line is the posterior mean\nand the dashed lines to either end denote the\npercentile- based 95% intervals.") +
theme_08 +
theme(legend.position = "none")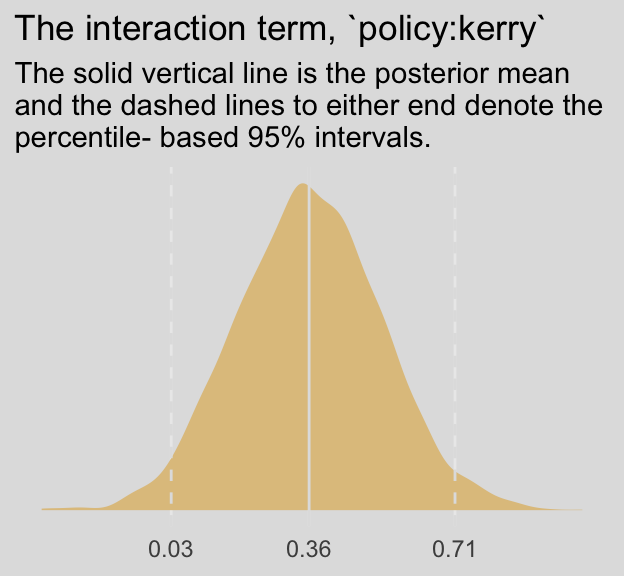
Following Hayes’s work on the bottom of page 295, here’s how you’d reproduce that by manipulating our \(\overline Y\) vectors.
post %>%
transmute(reproduced_interaction_term = (Y_bar_4 - Y_bar_3) - (Y_bar_2 - Y_bar_1)) %>%
mean_qi(reproduced_interaction_term) %>%
mutate_if(is.double, round, digits = 2)## # A tibble: 1 x 6
## reproduced_interaction_term .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 0.36 0.03 0.71 0.95 mean qiExtending that logic, we also get the answer this way.
post %>%
transmute(reproduced_interaction_term = (Y_bar_4 - Y_bar_2) - (Y_bar_3 - Y_bar_1)) %>%
mean_qi(reproduced_interaction_term) %>%
mutate_if(is.double, round, digits = 2)## # A tibble: 1 x 6
## reproduced_interaction_term .lower .upper .width .point .interval
## <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 0.36 0.03 0.71 0.95 mean qi8.4.1 Simple effects parameterization.
We might reacquaint ourselves with the formula from model8.5.
## interest ~ 1 + policy + kerry + policy:kerryThe results cohere nicely with the “Model 1” results at the top of Table 8.5.
## Estimate Est.Error Q2.5 Q97.5
## Intercept 1.781 0.089 1.611 1.961
## policy -0.382 0.124 -0.631 -0.142
## kerry 0.601 0.127 0.345 0.845
## policy:kerry 0.359 0.174 0.026 0.712The Bayesian \(R^2\) portion looks on point, too.
## Estimate Est.Error Q2.5 Q97.5
## R2 0.14 0.025 0.092 0.19Our various Y_bar transformations from before continue to cohere with the coefficients, above, just like in the text. E.g., the policy coefficient may be returned like so.
post %>%
transmute(b1 = b_policy,
`Y_bar_2 - Y_bar_1` = Y_bar_2 - Y_bar_1) %>%
pivot_longer(everything()) %>%
group_by(name) %>%
summarize(mean = mean(value),
sd = sd(value)) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 3
## name mean sd
## <chr> <dbl> <dbl>
## 1 b1 -0.382 0.124
## 2 Y_bar_2 - Y_bar_1 -0.382 0.124We can continue to use Hayes’s Y_bar transformations to return the kerry coefficient, too.
post %>%
transmute(b2 = b_kerry,
`Y_bar_3 - Y_bar_1` = Y_bar_3 - Y_bar_1) %>%
pivot_longer(everything()) %>%
group_by(name) %>%
summarize(mean = mean(value),
sd = sd(value)) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 3
## name mean sd
## <chr> <dbl> <dbl>
## 1 b2 0.601 0.127
## 2 Y_bar_3 - Y_bar_1 0.601 0.127Here we compute \(b_3\) with the difference between the simple effects of \(X\) at levels of \(W\).
post %>%
transmute(b3 = `b_policy:kerry`,
`(Y_bar_4 - Y_bar_3) - (Y_bar_2 - Y_bar_1)` = (Y_bar_4 - Y_bar_3) - (Y_bar_2 - Y_bar_1)) %>%
pivot_longer(everything()) %>%
group_by(name) %>%
summarize(mean = mean(value),
sd = sd(value)) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 3
## name mean sd
## <chr> <dbl> <dbl>
## 1 (Y_bar_4 - Y_bar_3) - (Y_bar_2 - Y_bar_1) 0.359 0.174
## 2 b3 0.359 0.174And now \(b_{3}\) with the difference between the simple effects of \(W\) at levels of \(X\).
post %>%
transmute(b3 = `b_policy:kerry`,
`(Y_bar_4 - Y_bar_2) - (Y_bar_3 - Y_bar_1)` = (Y_bar_4 - Y_bar_2) - (Y_bar_3 - Y_bar_1)) %>%
pivot_longer(everything()) %>%
group_by(name) %>%
summarize(mean = mean(value),
sd = sd(value)) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 3
## name mean sd
## <chr> <dbl> <dbl>
## 1 (Y_bar_4 - Y_bar_2) - (Y_bar_3 - Y_bar_1) 0.359 0.174
## 2 b3 0.359 0.1748.4.2 Main effects parameterization.
A nice feature of brms is you can transform your data right within the brm() or update() functions. Here we’ll make our two new main-effects-coded variables, policy_me and kerry_me, with the mutate() function right within update().
model8.6 <-
update(model8.5,
newdata = caskets %>%
mutate(policy_me = policy - .5,
kerry_me = kerry - .5),
family = gaussian,
interest ~ 1 + policy_me + kerry_me + policy_me:kerry_me,
cores = 4)Transforming your data within the brms functions won’t change the original data structure. However, brms will save the data used to fit the model within the brm() object. You can access that data like so.
## interest policy_me kerry_me
## 1 4 0.5 0.5
## 2 2 -0.5 0.5
## 3 3 0.5 0.5
## 4 1 0.5 0.5
## 5 1 0.5 0.5
## 6 2 -0.5 -0.5But we digress. Here’s our analogue to the “Model 2” portion of Table 8.5.
## Estimate Est.Error Q2.5 Q97.5
## Intercept 1.981 0.045 1.893 2.070
## policy_me -0.208 0.090 -0.387 -0.032
## kerry_me 0.780 0.090 0.607 0.955
## policy_me:kerry_me 0.354 0.180 0.007 0.707
## R2 0.140 0.025 0.093 0.191Like with model8.6, above, we’ll need a bit of algebra to compute our \(\overline Y_i\) vectors.
post <-
posterior_samples(model8.6) %>%
mutate(Y_bar_1 = b_Intercept + b_policy_me * -.5 + b_kerry_me * -.5 + `b_policy_me:kerry_me` * -.5 * -.5,
Y_bar_3 = b_Intercept + b_policy_me * -.5 + b_kerry_me * .5 + `b_policy_me:kerry_me` * -.5 * .5,
Y_bar_2 = b_Intercept + b_policy_me * .5 + b_kerry_me * -.5 + `b_policy_me:kerry_me` * .5 * -.5,
Y_bar_4 = b_Intercept + b_policy_me * .5 + b_kerry_me * .5 + `b_policy_me:kerry_me` * .5 * .5)With our post for fit5 in hand, we’ll follow the formulas at the top of page 298 to compute our \(b_1\) and \(b_2\) distributions.
post %>%
transmute(b1 = ((Y_bar_4 - Y_bar_3) + (Y_bar_2 - Y_bar_1)) / 2,
b2 = ((Y_bar_4 - Y_bar_2) + (Y_bar_3 - Y_bar_1)) / 2) %>%
pivot_longer(b1:b2) %>%
group_by(name) %>%
summarize(mean = mean(value),
sd = sd(value)) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 3
## name mean sd
## <chr> <dbl> <dbl>
## 1 b1 -0.208 0.09
## 2 b2 0.78 0.09Hayes pointed out that the interaction effect, \(b_3\), is the same across models his OLS Models 1 and 2. This is largely true for our Bayesian HMC model8.5 and model8.6 models.
## Estimate Est.Error Q2.5 Q97.5
## 0.359 0.174 0.026 0.712## Estimate Est.Error Q2.5 Q97.5
## 0.354 0.180 0.007 0.707However, the results aren’t exactly the same because of simulation error. If you were working on a project requiring high precision, increase the number of posterior iterations. To demonstrate, here we’ll increase each chain’s post-warmup iteration count by an order of magnitude, resulting in 80,000 post-warmup iterations rather than the default 4,000.
model8.7 <-
update(model8.5,
chains = 4, cores = 4, warmup = 1000, iter = 21000)
model8.8 <-
update(model8.6,
chains = 4, cores = 4, warmup = 1000, iter = 21000)Now they’re quite a bit closer.
## Estimate Est.Error Q2.5 Q97.5
## 0.360 0.178 0.011 0.711## Estimate Est.Error Q2.5 Q97.5
## 0.360 0.178 0.011 0.709And before you get fixate on how there are still differences after 80,000 iterations, each, consider comparing the two density plots.
posterior_samples(model8.7) %>%
transmute(iteraction = `b_policy:kerry`) %>%
bind_rows(
posterior_samples(model8.8) %>%
transmute(iteraction = `b_policy_me:kerry_me`)
) %>%
mutate(model = rep(str_c("8.", 7:8), times = n() / 2)) %>%
ggplot(aes(x = iteraction, fill = model)) +
geom_density(size = 0, alpha = 1/2) +
scale_fill_manual(values = ochre_palettes[["olsen_qual"]][c(3, 6)]) +
scale_y_continuous(NULL, breaks = NULL) +
labs(title = "The interaction densities, by model",
subtitle = "Yes, they are indeed different. And yet that difference is so\ntrivial that we'd expect greater variability from measurement\nerror than we still have from simulation error.",
x = NULL) +
theme_08 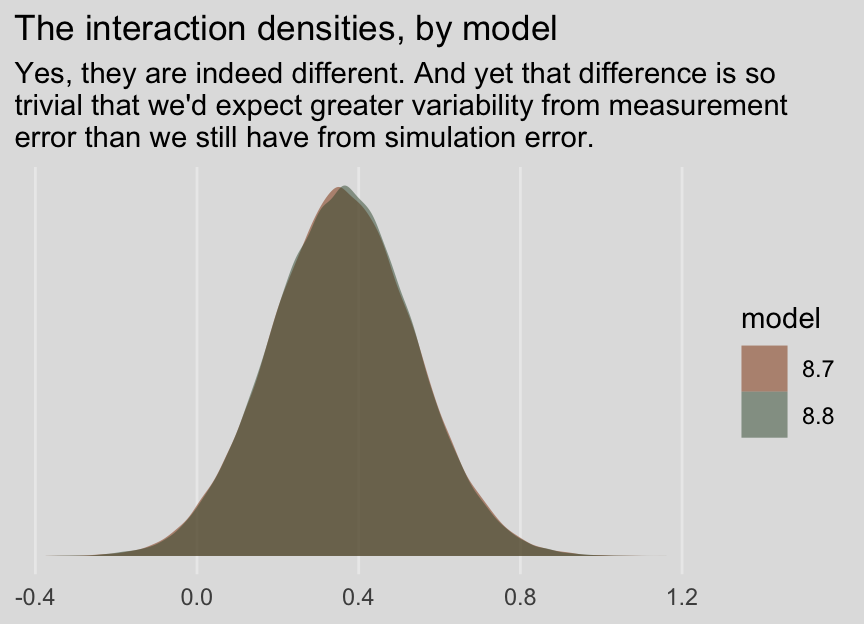
8.4.3 Conducting a \(2 \times 2\) between-participants factorial ANOVA using PROCESS another regression model with brms.
Since we’re square in single-level regression land with our brms approach, there’s no direct analogue for us, here. However, notice the post-ANOVA \(t\)-tests Hayes presented on page 300. If we just want to consider the \(2 \times 2\) structure of our two dummy variables as indicative of four groups, we have one more coding system available for the job. With the handy str_c() function, we’ll concatenate the policy and kerry values into a nominal variable, policy_kerry. Here’s what that looks like:
## # A tibble: 6 x 8
## policy interest age educ male conserv kerry policy_kerry
## <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <chr>
## 1 1 4 39 3 1 4 1 11
## 2 0 2 57 3 1 3 1 01
## 3 1 3 63 2 0 6 1 11
## 4 1 1 56 5 1 3 1 11
## 5 1 1 50 3 0 3 1 11
## 6 0 2 87 2 1 5 0 00Now check out what happens if we reformat our formula to interest ~ 0 + policy_kerry.
The brm() function recnognized policy_kerry was a character vector and treated it as a nominal variable. The 0 + part of the function removed the model intercept. Here’s how that effects the output.
## Family: gaussian
## Links: mu = identity; sigma = identity
## Formula: interest ~ 0 + policy_kerry
## Data: caskets (Number of observations: 541)
## Samples: 4 chains, each with iter = 2000; warmup = 1000; thin = 1;
## total post-warmup samples = 4000
##
## Population-Level Effects:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## policy_kerry00 1.78 0.09 1.61 1.96 1.00 4728 3033
## policy_kerry01 2.38 0.09 2.21 2.56 1.00 4673 3337
## policy_kerry10 1.40 0.09 1.22 1.57 1.00 5379 3114
## policy_kerry11 2.36 0.09 2.19 2.52 1.00 4375 2931
##
## Family Specific Parameters:
## Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
## sigma 1.04 0.03 0.98 1.10 1.00 4792 2939
##
## Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
## is a crude measure of effective sample size, and Rhat is the potential
## scale reduction factor on split chains (at convergence, Rhat = 1).Without the typical intercept, brm() estimated the means for each of the four policy_kerry groups. It’s kinda like an intercept-only model, but with four intercepts. Here’s what their densities look like:
post <- posterior_samples(model8.9)
post %>%
pivot_longer(b_policy_kerry00:b_policy_kerry11) %>%
mutate(name = str_remove(name, "b_")) %>%
ggplot(aes(x = value, fill = name)) +
geom_density(color = "transparent", alpha = 2/3) +
scale_fill_manual(NULL,
values = ochre_palettes[["olsen_qual"]][c(5, 6, 4, 3)]) +
scale_y_continuous(NULL, breaks = NULL) +
labs(title = "Group means",
x = NULL) +
theme_08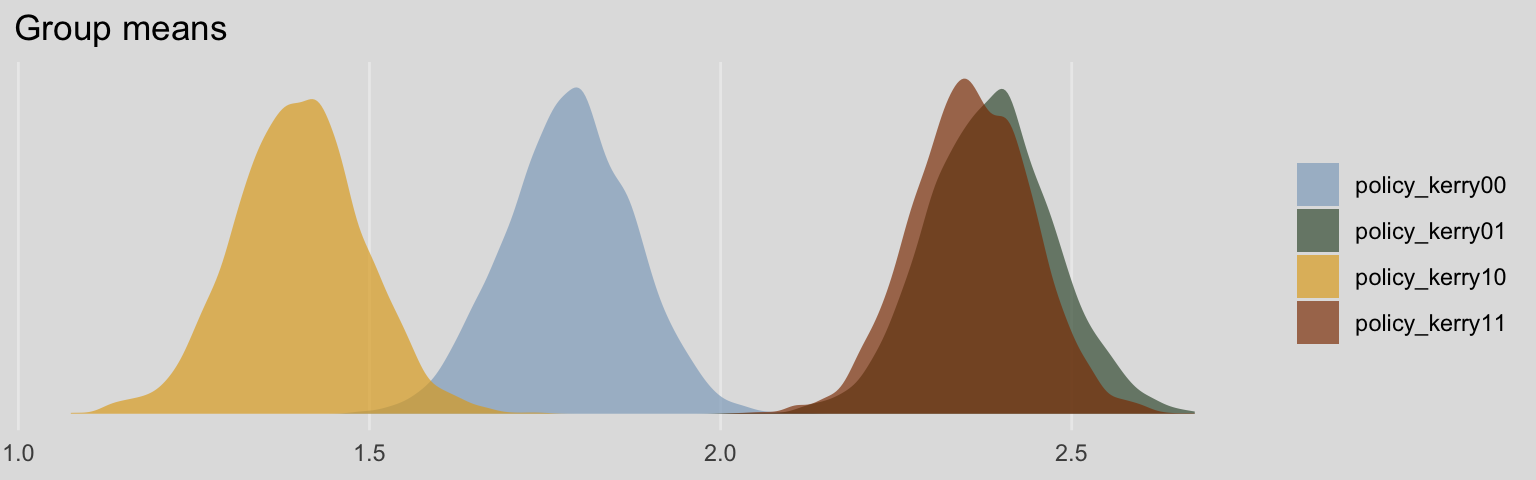
Since each of the four primary vectors in our post object is of a group mean, it’s trivial to compute difference scores. To compute the difference score analogous to Hayes’s two \(t\)-tests, we’d do the following.
post %>%
mutate(difference_1 = b_policy_kerry10 - b_policy_kerry00,
difference_2 = b_policy_kerry11 - b_policy_kerry01) %>%
pivot_longer(starts_with("difference_")) %>%
group_by(name) %>%
median_qi(value) %>%
mutate_if(is.double, round, digits = 3)## # A tibble: 2 x 7
## name value .lower .upper .width .point .interval
## <chr> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
## 1 difference_1 -0.386 -0.639 -0.137 0.95 median qi
## 2 difference_2 -0.026 -0.273 0.213 0.95 median qiSession info
## R version 3.6.0 (2019-04-26)
## Platform: x86_64-apple-darwin15.6.0 (64-bit)
## Running under: macOS High Sierra 10.13.6
##
## Matrix products: default
## BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
## LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
##
## locale:
## [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] tidybayes_1.1.0 ochRe_1.0.0 ggthemes_4.2.0 brms_2.10.3 Rcpp_1.0.2 forcats_0.4.0
## [7] stringr_1.4.0 dplyr_0.8.3 purrr_0.3.3 readr_1.3.1 tidyr_1.0.0 tibble_2.1.3
## [13] ggplot2_3.2.1 tidyverse_1.2.1
##
## loaded via a namespace (and not attached):
## [1] colorspace_1.4-1 ellipsis_0.3.0 ggridges_0.5.1
## [4] rsconnect_0.8.15 ggstance_0.3.2 markdown_1.1
## [7] base64enc_0.1-3 rstudioapi_0.10 rstan_2.19.2
## [10] svUnit_0.7-12 DT_0.9 fansi_0.4.0
## [13] lubridate_1.7.4 xml2_1.2.0 codetools_0.2-16
## [16] bridgesampling_0.7-2 knitr_1.23 shinythemes_1.1.2
## [19] zeallot_0.1.0 bayesplot_1.7.0 jsonlite_1.6
## [22] broom_0.5.2 shiny_1.3.2 compiler_3.6.0
## [25] httr_1.4.0 backports_1.1.5 assertthat_0.2.1
## [28] Matrix_1.2-17 lazyeval_0.2.2 cli_1.1.0
## [31] later_1.0.0 htmltools_0.4.0 prettyunits_1.0.2
## [34] tools_3.6.0 igraph_1.2.4.1 coda_0.19-3
## [37] gtable_0.3.0 glue_1.3.1.9000 reshape2_1.4.3
## [40] raster_2.9-5 cellranger_1.1.0 vctrs_0.2.0
## [43] nlme_3.1-139 crosstalk_1.0.0 xfun_0.10
## [46] ps_1.3.0 rvest_0.3.4 mime_0.7
## [49] miniUI_0.1.1.1 lifecycle_0.1.0 gtools_3.8.1
## [52] zoo_1.8-6 scales_1.0.0 colourpicker_1.0
## [55] hms_0.4.2 promises_1.1.0 Brobdingnag_1.2-6
## [58] parallel_3.6.0 inline_0.3.15 shinystan_2.5.0
## [61] gridExtra_2.3 loo_2.1.0 StanHeaders_2.19.0
## [64] stringi_1.4.3 dygraphs_1.1.1.6 pkgbuild_1.0.5
## [67] rlang_0.4.1 pkgconfig_2.0.3 matrixStats_0.55.0
## [70] evaluate_0.14 lattice_0.20-38 rstantools_2.0.0
## [73] htmlwidgets_1.5 labeling_0.3 tidyselect_0.2.5
## [76] processx_3.4.1 plyr_1.8.4 magrittr_1.5
## [79] R6_2.4.0 generics_0.0.2 pillar_1.4.2
## [82] haven_2.1.0 withr_2.1.2 xts_0.11-2
## [85] sp_1.3-1 abind_1.4-5 modelr_0.1.4
## [88] crayon_1.3.4 arrayhelpers_1.0-20160527 utf8_1.1.4
## [91] rmarkdown_1.13 grid_3.6.0 readxl_1.3.1
## [94] callr_3.3.2 threejs_0.3.1 digest_0.6.21
## [97] xtable_1.8-4 httpuv_1.5.2 stats4_3.6.0
## [100] munsell_0.5.0 shinyjs_1.0