笔记 16 化学品与健康
16.1 Chemicals in our environment: What is a chemical, and how are we exposed?
16.1.1 Chemical defination
- A compound or substance that has been purified or prepared, especially artificially
16.1.2 How Populations Are Exposed to Chemicals

16.1.3 How Chemicals Get into Our Bodies

16.1.4 Database
16.1.5 The Chemical Industry Work Environment
16.1.6 Occupational Health: Understanding and Assessing Exposures and Outcomes
- Occupational Health Surveillance
- Exposure Assessment
Occupational Epidemiology Study Designs
- Cohort: a designated group of individuals who are identified as eligible for the study, subject to further inclusion factors, in order to evaluate factors associated with adverse health outcomes
- no random selection of individuals into the cohort
- Cohort studies can be prospective or retrospective
What Is the Healthy Worker Effect?
- Observation that workers exhibit lower mortality rates than the general population
- Selection of “healthier” persons into the workforce
- Retention of “healthier” persons in the workforce
- In appropriate comparison population that differs in terms of Vital status ascertainment Differential diagnoses Risk factors for mortality Access to treatment
16.1.7 Industrial Hygiene Practices
16.1.8 Regulatory Programs and Initiatives
16.2 Toxicology: What do chemicals do in our bodies?
16.2.1 Intro to Chemicals and Health
- Toxicology: the study of poisons
- Poisons: chemical or physical substances which produce adverse responses in biological organisms
- Toxicants: toxic substances that are either manmade or result from human activity
- Toxins: usually toxic substances produced by living organisms, such as reptiles, insects, plants, and microorganisms
“The dose makes the poison” — Paracelsus
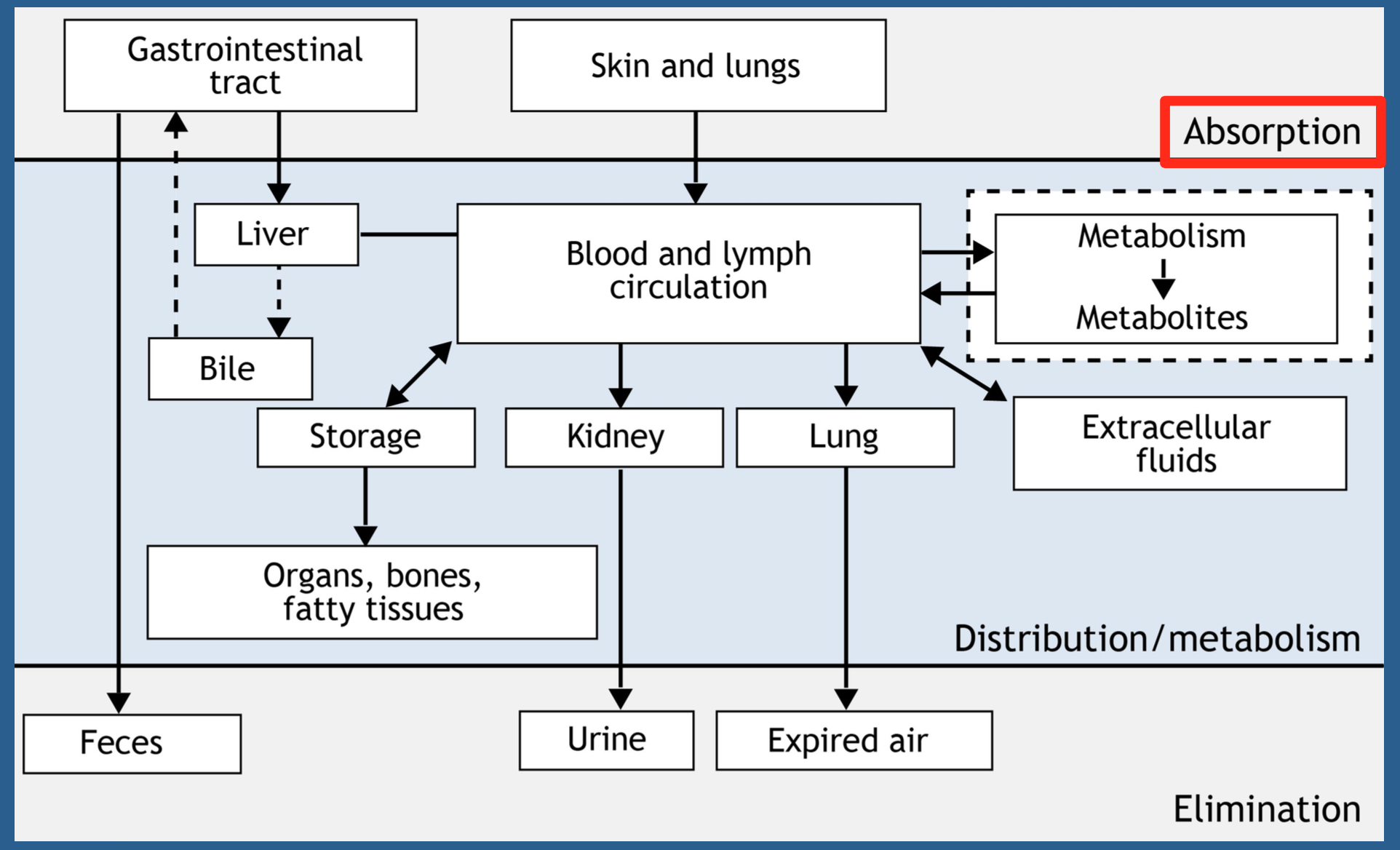
16.2.2 Processes Involved in Chemical Toxicity
- Exposure is outside the body
- After absorption, you have an internal dose
- Distribution mainly through blood
- Biotransformation
- Elimination
- Storage
- Fat (lipid-soluble)
- Bone (minerals)
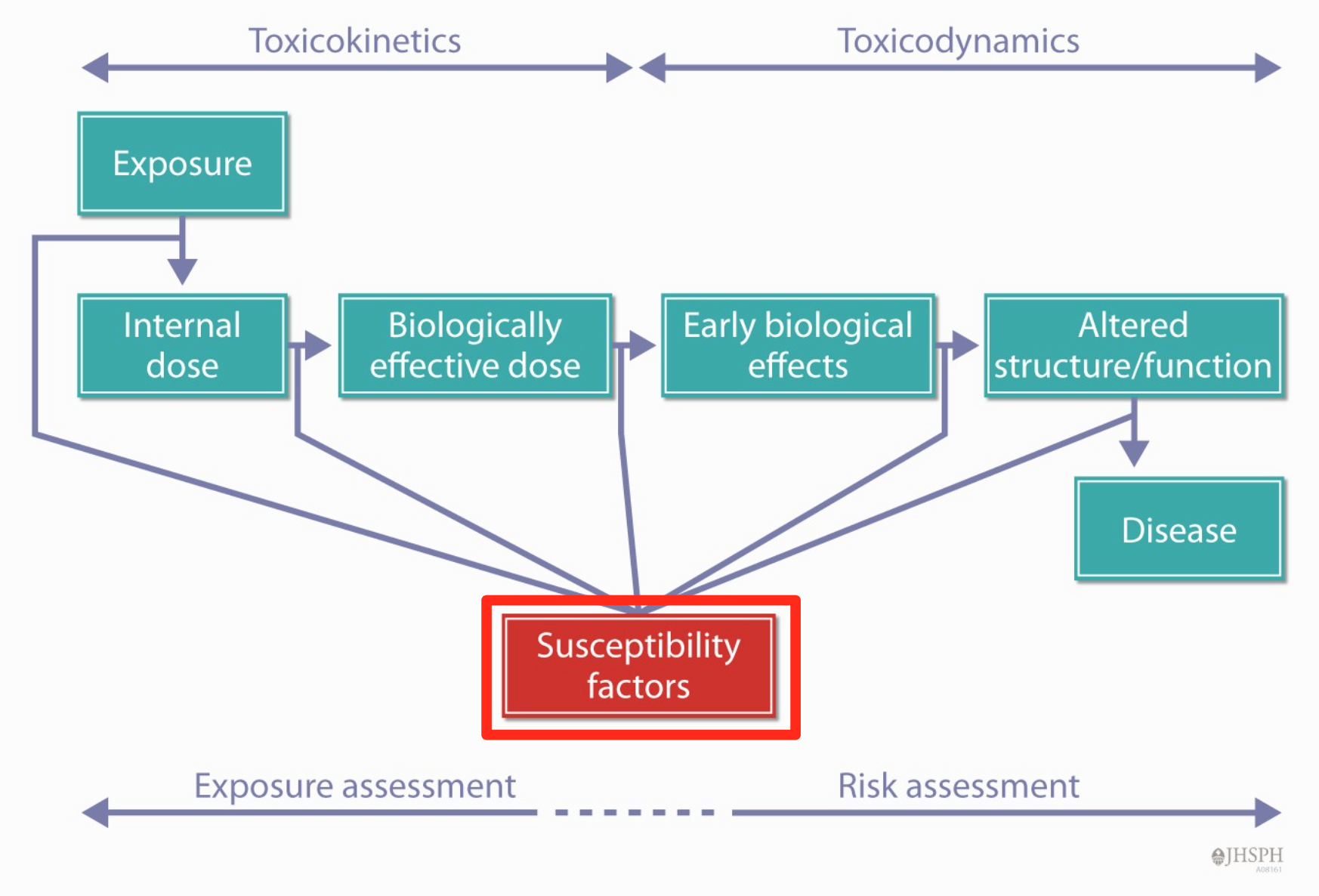
- The end result of these toxicokinetic processes is a biologically effective dose (BED) of the toxicant
- The biologically effective dose is that portion of the internal dose that interacts with biological molecules (targets)
- Leads to changes in cells
- Molecular
- Biochemical changes
- Cell toxicity
- Organ dysfunction/disease
- Leads to changes in cells
- The effect depends on the dose of the agent
- Toxicokinetics: (movement) processes that the body subjects the chemical to
- Absorption: taking in of a chemical from a site(s) of exposure (i.e., gastrointestinal system) resulting in an internal dose
- Distribution: distributing a chemical from its site of absorption throughout the body
- Excretion/elimination: removing the chemical from the body
- Biotransformation/metabolism: modification of chemicals by enzymes in the body
- Toxicodynamics: (changes) what the chemical does to the organism, Toxicodynamics refers to the effects of toxicants (or their metabolites) in biological systems
- Some interactions with target molecules result in an adverse biological effect, while others may not
- For some chemicals, the form that interacts with targets is the original chemical
- For other chemicals, the toxic form is a biotransformation product(s) of that chemical
16.3 Biomonitoring: How do we measure chemicals in our bodies and why?
16.3.1 Exposure Assessment Approaches
- Questionnaire/historical information
- Environmental monitoring
- Personal monitoring
- Biomonitoring
- Combine these approaches with calibrated and validated models
16.3.2 Biomonitoring
- A useful tool for exposure assessment
- Defined as the assessment of internal dose by measuring the parent chemical (or its metabolite or reaction product) in human specimens (for example: blood, urine)
- Measurement of concentrations, not exposures
16.3.3 Evaluation of Human Exposure Using Biomonitoring
- Large–scale population surveys
- NHANES: provides a foundation for CDC’s National Biomonitoring Program
- Epidemiological studies of specific populations
- Susceptible/vulnerable
- Highly exposed (for example: NICU infants, occupational settings)
16.3.4 Considerations in Biomonitoring
- What is the best chemical measure (analyte)?
- Parent chemical or metabolite or adduct
- What is the best time to obtain the specimen?
- Measurement time windows
- What is the best specimen (matrix)?
- Blood and urine
- Analytical considerations
- Related to the specimen (for example: stability, contamination, interferences, etc.)
- Related to the method (for example: validated method, sensitivity, specificity, QA/QC, etc.)
16.3.5 NHANES: How We Assess Exposure of the US Population to Priority Environmental Chemicals
- Began in 1971
- Continuous survey since 1999 (survey cycle = two years)
- Stratified, multistate national probability sample
- About 10,000 participants in 30 locations every two years
- method
- Face-to-face and computer-assisted interviews:Demographics, Socioeconomic, Dietary,Health-related topics
- Physical examination
- Biological specimen collection
- More at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- Not all chemicals are measured in everyone, except:Blood Pb, Cd, and Hg: all persons one year and older, Serum cotinine: all persons three years and older
- Most urinary chemical measurements are made in a 1/3 subsample of the participants, Ages six years and older,Subsamples are determined so they are representative of the US population
- Biomonitoring results are presented in the National Report on Human Exposure to Environmental Chemicals
- DATA
- Chemical properties (for example: persistence in the body, metabolism, stability) and analytical considerations are factors in biomarker selection and specimen collection
- Detection of a chemical does not mean that it causes disease
16.3.6 Biomonitoring at the state and local laboratories
- CDC’s data can not be used at the state & local level
- APHL. Public health laboratories
16.4 Health effects of chemicals: How do we figure out how chemicals affect our health?
16.4.1 Discuss different types of health effects
- Acute vs latent
- Transient vs chronic
- Beneficial vs adverse
16.4.2 Describe various health endpoints
- Acute toxicity
- Generally related to short-term exposure,severeeffects
- Example:carbonmonoxidepoisoning
- Can have short-term and long-term consequences
- Repeated dose toxicity -Depends on toxicokinetics(what happens to the toxin in the body, and how quickly)
- Effects could be acute(threshold),subacute,orchronic
- Example: some medications build up effects(e.g.,acetaminophen)
- Genotoxicity
- Some chemicals can damage DNA, causing mutations when the cells divide
- These mutations can lead to cancer if the cells are in not reproductive(eggorsperm) cells, or to some birth defects
- Carcinogenicity
- Ability to cause cancer or make cancer more likely
- Can be director in direct effects
- Most chemicals that have been identified as likely carcinogens have been from animal studies; fewer from direct human epidemiology
- Occupational workers most likely to be exposed; examples are coke oven workers, asbestos workers
- Reproductive toxicity
- Reproduction is complex, and process can be interrupted at many points
- Some effects from direct toxicity to egg or sperm cells, other effects on developing
- Difficult to identify in people; most identified in animal models fetus
- Endocrine disruption
- Relatively recent findings of chemicals mimicking naturally occurring hormones such as sex hormones
- Possible links to development, maturation, sexual maturation
- Early in our understanding
- Neurotoxicity: general,delayed
- Effect on developing or mature nervous system
- Effects on central nervous system including cognition(lead), peripheral nervous system
- Other: immunotoxicity, allergenicity, intolerance
- Toxicity endpoints
- National Environmental Health Tracking Network
- Causal association: Hill’s Causal Criteria
16.4.3 Risk Assessment in Environmental Decision Making
- Risk Assessment
- a policy tool designed to facilitate management of environmental health hazards
- Risk assessment is an integration of information from various scientific domains
- facilitate decision making, not science
- Who Uses Risk Assessment
- Federal and international agencies
- State environmental/health agencies
- Industry
- Non-governmental groups ### The Four Steps of Risk Assessment
- Hazard Identification
- Identify chemicals of concern and potential associated health effects
- Evaluate evidence that exposure to a given chemical elicits an adverse response
- Qualitative evaluation of relationship between exposure and adverse effects
- Effects seen in one human population are predictive for others
- Average human may be as sensitive as most sensitive animal responder
- Site concordance (tumors) or effects concordance (non-cancer responses) do not necessarily hold
- Dose-Response Assessment
- Quantitative characterization of the relationship between a chemical agent dose and the incidence of adverse health effects
- Where possible, quantitative toxicity metrics are presented
- Exposure Assessment
- Quantitative characterization of human exposure/contact with chemical hazards
- Integrates information on Chemical concentrations in environmental media, Human activity patterns and Population characteristics
- Predicts magnitude of human exposure in the form of external dose in milligrams per kilogram of body weight per day
- Estimation of intake of a chemical
- Key components Concentration of chemical in media of interest, Media intake rate, Time components(Frequency Duration Averaging time) and Body weight
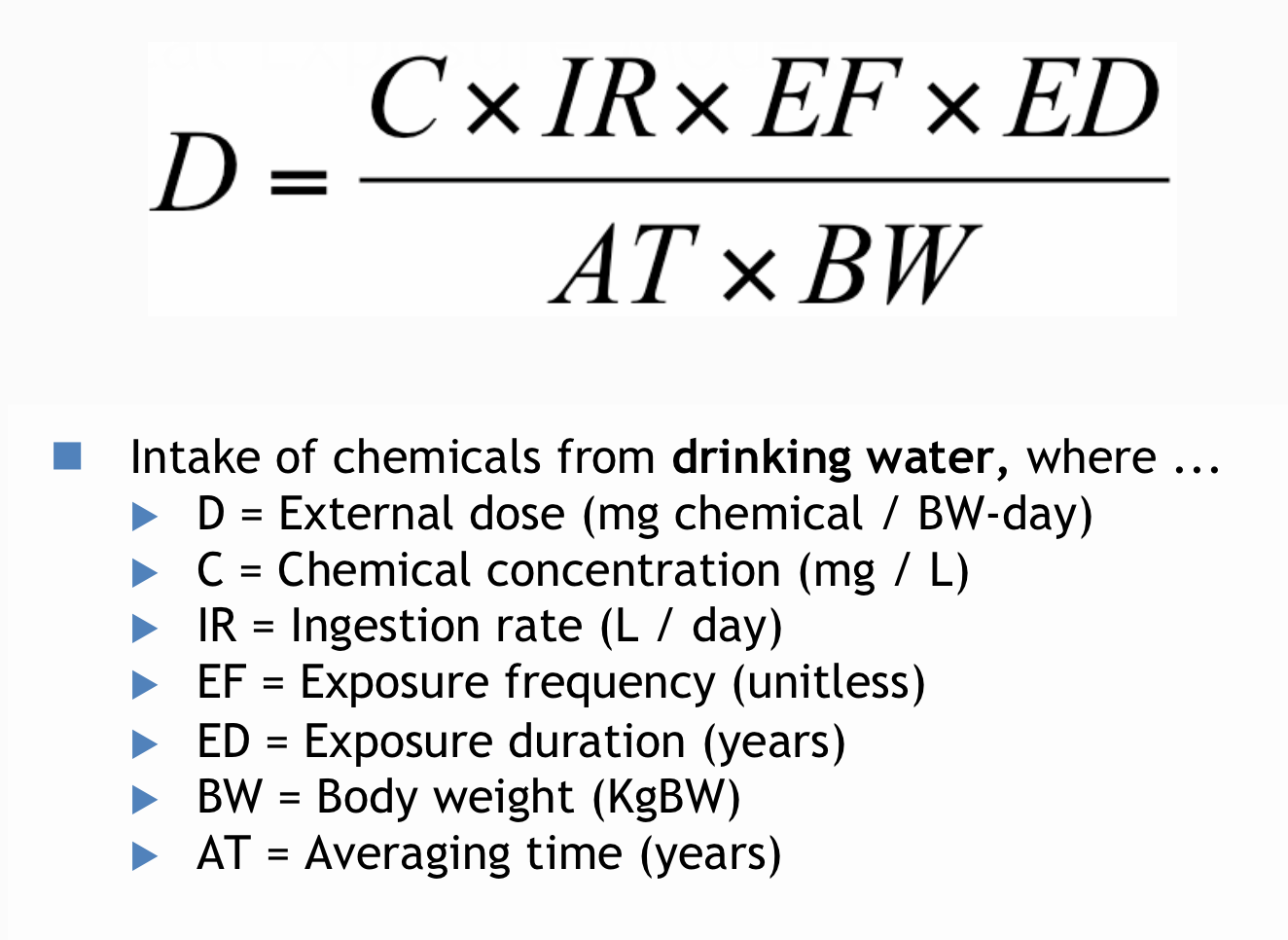
Risk Characterization
- Integration of information from hazard identification, dose-response evaluation, and exposure assessment steps
- A risk characterization often includes …
- Prediction of carcinogenic risks and potential for non-carcinogenic hazard
- Estimation of population burden (cancer)
- Uncertainty discussion
- Quantitative Risk Characterization
- Risk of 1 x 10e-6 is “one case per million persons exposed over a lifetime”
- Risk = exposed dose × effect unitless
- No. cases = risk × population size
- The outputs of a risk assessment typically include an estimate of cancer risk, non- cancer hazard, population cancer burden, and an uncertainty discussion
16.5 Chemicals policy: What do we do about chemicals and health?
16.5.1 Toxic Substances Control Act
- Covers most chemicals used in industry and in commercial/consumer products
- Excludes:
- Uses in drugs, cosmetics, food, and food packaging regulated by FDA
- Uses in pesticides covered by EPA under FIFRA
- Basic provisions have never been amended
- Chemical Policy
- Legislation
- Regulation
- Court decisions (“case law”)
16.5.2 Drivers for Chemical Policy Reform
- Chemicals Are Ubiquitous
- 10 trillion pounds produced per year in the US
- Used to make 96% of all materials and products
- Large but unknown number of chemicals in US commerce
- Science Drivers: Connecting the Dots
- Certain chronic diseases are on the rise
- Certain chemicals are linked to those same chronic diseases
- Many of those same chemicals are in us
- Diseases Linked to Chemical Exposures
- Cancer
- Learning and developmental disabilities
- Parkinson’s and Alzheimer’s disease
- Reproductive health and fertility problems
- Asthma
- Diabetes
- Obesity
- Immune disorders
- Cardiovascular disease
- Understanding of extent and pathways of chemical exposures
- Long-range transport
- Migration of chemicals from products into environment
- Disproportionate exposures: environmental justice issues
- Advent of biomonitoring
- Early-life exposures
- Risk assessment evolution and controversy
- “RedBook”(1983),and a major update “SilverBook”(2009)
- Key challenges: Human variability, Uncertainty, Cumulative effects and exposures(Multiple chemicals, Chemicals and other stressors)
- Emerging high-throughput testing: Tox21
16.5.3 Why Legislative Reform?
- New chemicals
- No data, no problem
- Guessing game
- Catch-22
- Anti-precaution
- Why Now?
- State legislation and policy changes
- Top priority of last two EPA administrators
- Market demand, especially from downstream users
- Retail regulation
- European Union’s REACH Regulation and Canadian Environmental Protection Act
- REACH
- “No data,no market”
- Shifting the burden of proof: Industry required to show safety
- Information flow in supply chains: Two-way flow between suppliers <–> customers
- Authorization required to use substances of very high concern
- Action