2.7 Placebo effect and using controls
What if people thought they were on the wholegrain diet, but they weren't? Perhaps surprisingly, individuals in a study may report effects of a treatment (either positive or negative), even if they have not received an active treatment. This could also compromise the internal validity of the study.
This is called the placebo effect.
Managing the placebo effect is difficult! However, impact of the placebo effect can be minimized using a control group: units of analysis without the treatment applied, but as similar as possible in every other way to those units of analysis receiving the treatment. This allows the effect of the treatment to be ssessed, over and above the placebo effect.
Sometimes the control group receives a placebo. A placebo is a non-effective treatment. Those who receive the placebo should be selected through random allocation when possible. Sometimes, using a placebo is unethical. The Wikipedia entry about placebos is intriguing.
![]()
Example 2.15 (Placebo effect) In the Himalaya 292 study, the authors report
That is, the subjects were blinded to the diet they were exposed to. However, some may think they are on the refined cereal or Himalaya diet, and respond accordingly (perhaps unconsciously).On each day of the intervention periods, volunteers were asked to consume a combination of bread, breakfast cereal, muffins and crackers that would supply in total 103g of the test cereal. The aim was for each volunteer to consume 60g cereal flakes (or puffed rice for the refined cereal diet), two slices of bread, one muffin and six savoury crackers each day. Volunteers were not told the identity of the test cereal in the foods provided to them
--- (Bird et al. (2008), p. 1033)
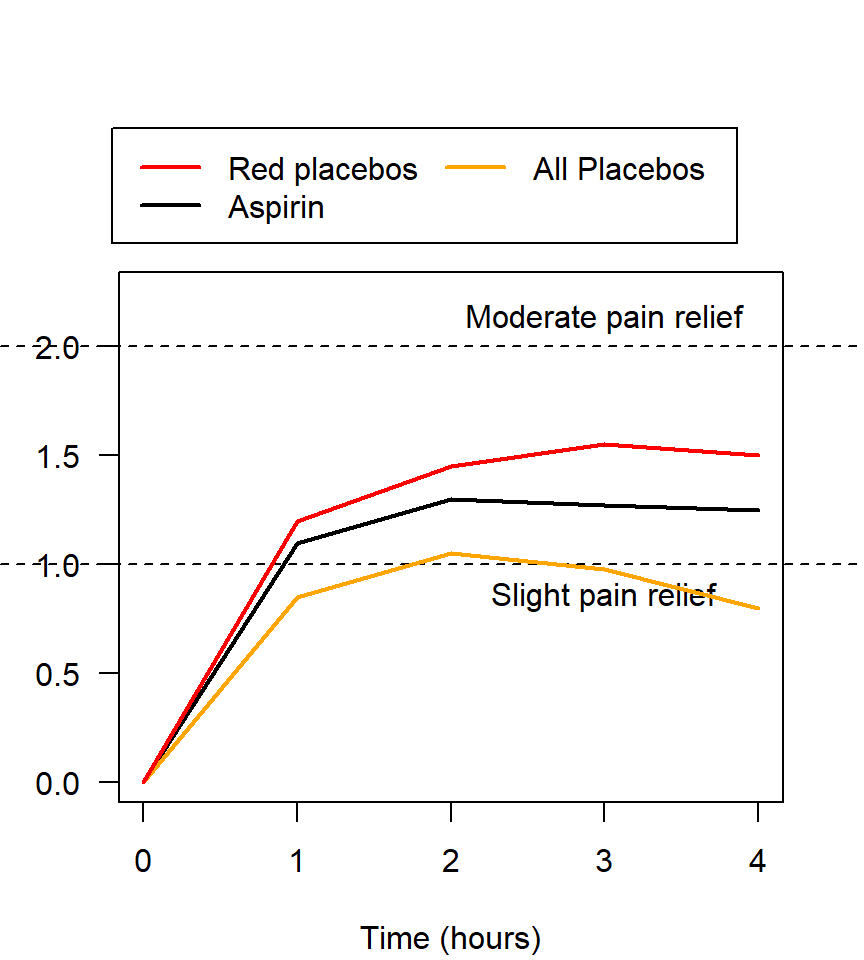
Figure 2.6: Pain relief, for various pain relief medicine
Example 2.17 (Placebo effect) A study of placebos (Waber et al. 2008) gave half the subjects a placebo, but told them that the pill was an expensive (implying 'very effective') pain killer ($2.50 per tablet). The other half were also given a placebo, but were told that the pill was a discount (impling 'less effective') pain killer ($0.10 per tablet).
About 85% of participants in the first group reported a pain reduction, yet only 61% in the second group reported a pain reduction. Remember that both groups actually received a placebo!